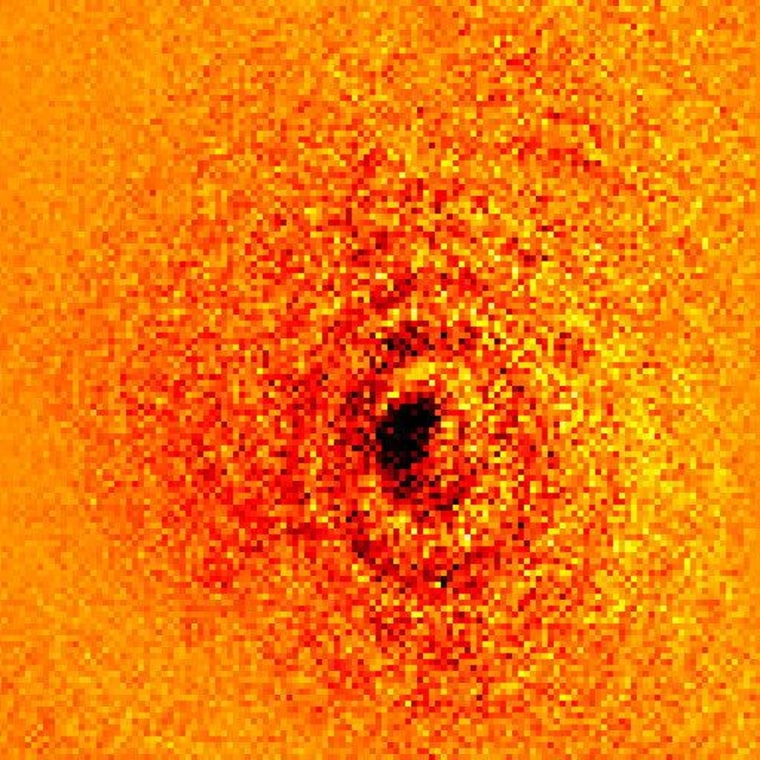
Capturing an image of an atom is difficult enough, but an atom's shadow? Considering that the wavelengths of visible light dwarf the size of atoms by orders of magnitude, one might think that it wouldn't even be possible for an atom to cast a shadow. Yet researchers at Griffith University in Australia have done both with a high-precision laser and sensor, producing a striking silhouette of a single ytterbium atom.
Professor Dave Kielpinski of Griffith's Center for Quantum Dynamics explained the significance of the feat in a news release accompanying the publication of their paper: "We have reached the extreme limit of microscopy. ... If we change the frequency of the light we shine on the atom by just one part in a billion, the image can no longer be seen."
It was accomplished by firing a microscopic laser beam at a single atom of ytterbium, which was suspended electrically in a vacuum. Most wavelengths of light would pass right by the atom without interacting with it, but the team found an incredibly specific wavelength that would be absorbed by ytterbium, and set out to make that atom cast a shadow in a way they could capture.
Why ytterbium? Kielpinski explained in an email to msnbc.com: "For many atomic elements, the correct lasers are very hard to build. The lasers we need to experiment on ytterbium ions are relatively simple and cheap. However, any atom would cast a shadow if you use the right color of laser."
The length of this "exposure" is about one second, and any particle physicist can tell you that getting an atom to sit still for a full second while you fire a laser at it is no small matter. The team thinks they can get the exposure time down to a fifth of a second, which may improve the accuracy of the image.
You may notice that in the image itself, there are concentric rings around the central shadow. These are not electron orbitals, like those you might see in a diagram of the same atom; it's just a normal side effect of how light travels around such a tiny object.
Erik Streed, the other lead member of the research team, explains that the technique could be useful to quantum computing or biomicroscopy: "We can now predict how much light is needed to observe processes within cells, under optimum microscopy conditions, without crossing the threshold and destroying them." Knowing that is very important, since an excess of energy delivered by light could alter processes on a molecular level.
More small-scale imaging:
- Itsy-bitsy structure in shape of Olympic rings created
- Microscope makes 3-D images of nano structures
- A microscope that can see atoms
- Naked molecule exposed
The paper, "Absorption Imaging of a Single Atom," was published today in Nature Communications. Andreas Jechow and Benjamin Norton also contributed to the research.
Devin Coldewey is a contributing writer for msnbc.com. His personal website is coldewey.cc.