Abstract
Respiratory tract infections with viruses and Pasteurella spp. were determined sequentially among 26 cattle that died during two severe epizootics of shipping fever pneumonia. Nasal swab and serum samples were collected prior to onset of the epizootics, during disease progression, and after death, when necropsies were performed and lung samples were collected. Eighteen normal control cattle also were sampled at the beginning of the epizootics as well as at weekly intervals for 4 weeks. Respiratory bovine coronaviruses (RBCV) were isolated from nasal secretions of 21 and 25 cattle before and after transport. Two and 17 cattle nasally shed Pasteurella spp. before and after transport, respectively. RBCV were isolated at titers of 1 × 103 to 1.2 × 107 PFU per g of lung tissue from 18 cattle that died within 7 days of the epizootics, but not from the lungs of the remaining cattle that died on days 9 to 36. Twenty-five of the 26 lung samples were positive for Pasteurella spp., and their CFU ranged between 4.0 × 105 and 2.3 × 109 per g. Acute and subacute exudative, necrotizing lobar pneumonia characterized the lung lesions of these cattle with a majority of pneumonic lung lobes exhibiting fibronecrotic and exudative changes typical of pneumonic pasteurellosis, but other lung lobules had histological changes consisting of bronchiolitis and alveolitis typical of virus-induced changes. These cattle were immunologically naive to both infectious agents at the onset of the epizootics, but those that died after day 7 had rising antibody titers against RBCV and Pasteurella haemolytica. In contrast, the 18 clinically normal and RBCV isolation-negative cattle had high hemagglutinin inhibition antibody titers to RBCV from the beginning, while their antibody responses to P. haemolytica antigens were delayed. Evans' criteria for causation were applied to our findings because of the multifactorial nature of shipping fever pneumonia. This analysis identified RBCV as the primary inciting cause in these two epizootics. These viruses were previously not recognized as a causative agent in this complex respiratory tract disease of cattle.
Shipping fever pneumonia (SFP) occurs frequently among cattle after transport and is characterized by fever, dyspnea, and exudative inflammatory and necrotizing lung lesions (11, 37). This form of pneumonia affected 64% of fatal cases in a study of feedlot cattle in Colorado (17). A multifactorial etiological concept for SFP is widely accepted in scientific circles, which implies that crowding and other stressful conditions favor virus spread and infections of respiratory tracts that become complicated with Pasteurella and other bacterial infections, often leading to fatal pneumonia (13, 37). Losses from SFP continue to occur in spite of widespread use of modern management and vaccination programs derived from decades of intensive research on physiological factors, infectious agents and pathogenesis of respiratory tract diseases, defense mechanisms and immune responses of cattle, modern vaccines, metaphylactic and therapeutic antibiotic treatments, and improved diagnostic tools (30, 37).
Viruses currently considered as potential etiological factors of SFP include bovine herpesvirus-1 (BHV-1) of infectious bovine rhinotracheitis (18, 23, 37), bovine parainfluenza type-3 virus (PI-3) (2, 26, 27), bovine respiratory syncytial virus (BRSV) (5, 36, 37), and bovine viral diarrhea virus (BVDV) (25, 37). Respiratory tract samples yielded BHV-1 and no other viruses in 18% of 354 fatal cases of SFP in an investigation reported by Jensen and coworkers (17). Isolations of BHV-1 were made from nasal or eye secretions and tracheal samples, but rarely from affected lungs (17, 18, 24). Infections with PI-3 were detected sporadically in lungs of field cases of SFP (26, 37). The direct involvement of BRSV or BVDV in naturally occurring epizootics of SFP has not been documented in published reports (37). Experimental exposure of calves to BRSV induced respiratory distress (5, 36), but this virus was reisolated only from nasal swab samples, and bacterial infections were detected in the lungs of 8 of 12 experimental animals (36). Sequential inoculations of cattle with BHV-1, PI-3, or BVDV and Pasteurella haemolytica induced more severe signs of clinical disease than the single infections (2, 18, 25, 27). Bacterial insults in SFP are P. haemolytica or Pasteurella multocida as well as Haemophilus somnus infections (4, 6, 14, 35).
Respiratory bovine coronaviruses (RBCV) were first isolated in 1993, and they were identified as emerging respiratory tract infections of cattle after transport to feed yards (28). Infections with RBCV had not been considered in the past as an etiological factor in SFP of cattle (37). High rates of primary respiratory tract infections with this virus and secondary infections with Pasteurella spp. among 105 and 120 cattle during two severe SFP epizootics were reported (21, 30). The objectives of the current investigations were to examine nasal shedding of viruses and P. haemolytica or P. multocida during the pathogenesis of fatal SFP among 26 cattle under experimentally designed conditions, to quantitate the infectious loads of these viruses and bacteria in the lungs, to relate these infections to the pneumonic lung lesions, to compare antibody responses to RBCV and P. haemolytica between fatal cases and clinically normal control cattle that remained RBCV isolation negative during these epizootics, and to analyze the etiological roles of the newly emerging RBCV infections according to Evans' criteria for causation (7).
MATERIALS AND METHODS
Experimental design.
Ten of 105 and 16 of 120 cattle of two SFP epizootics occurring in 1997 and 1998 (97TXSF and 98TXSF, respectively) were subjected to prospective, experimentally designed sampling and testing at the time of assembly (day 0), after transport (day 5), and throughout the pathogenesis of fatal pneumonia. The cattle were ear-tagged and clinically examined at assembly, when initial sets of nasal swab samples for culture of infectious agents and blood samples for serum harvest were collected. Nasal swab samples were placed into transport medium for virus isolation, while dry samples were used for bacterial cultures. Nasal swab samples were placed on dry ice and subsequently transferred into −70°C freezers. All cattle were vaccinated with modified-live virus vaccines against BHV-1 and PI-3 in 1997, and only against BHV-1 in 1998 (Prevail and Reliant; Rhone Merieux, Inc., Athens, Ga.). The experimental calves also were given blackleg vaccines (Electroid 7; Mallinckrodt Vet. Inc., Mundelein, Ill.) and ivermectin (Ivomec; Merck and Co., Inc., Rahway, N.J.). They remained for 4 days at the assembly site of the order buyer and then were transported 1,932 km to the feed yard in Bushland, Tex., jointly operated by the Agricultural Research Service and the Texas Agricultural Experiment Station. They were clinically examined on the day of arrival in the feed yard (day 5) and daily thereafter. Nasal swab and blood samples also were taken at this time and subsequently on a weekly schedule. Seven and 11 cattle of the respective epizootics remained clinically normal and were also tested microbiologically and serologically as contact controls.
Necropsies, pathological, histopathological, and immunocytochemical examinations.
Necropsies were performed immediately after death, and lung samples were collected at the Texas Veterinary Diagnostic Laboratory in Amarillo, Tex. Aliquots of the lung samples were frozen at −70°C for isolation, identification, and quantification of viruses and bacteria. Pieces from each lung were used to make exfoliative cytological or cryostat preparations for immunofluorescent tests with antibodies specific for RBCV and BVDV. Lung tissue pieces were placed into neutral 10% phosphate-buffered formalin for fixation. Histological sections were prepared and stained by the hematoxylin-eosin methods.
Bacterial isolation, identification, and quantification.
Dry nasal swab samples were removed from the freezer and allowed to thaw at 25°C. Each swab was streaked over one-fourth of the surface of a plate. The lung specimens were thawed, the surfaces were seared with a hot spatula, and a 1-g sample was removed from the center of each specimen. Lung tissues were minced with scissors and homogenized in phosphate-buffered saline (PBS) at pH 7.4 by grinding with a Ten Broeck device. The resulting 10% (wt/vol) suspension was serially diluted in 10-fold steps. Aliquots of 0.1 ml of the dilutions were plated in five replicas on tryptose agar plates fortified with 5% citrated bovine blood to enumerate CFU. The culture plates were incubated at 37°C for 24 h in an atmosphere with 5% CO2. Bacterial colonies were counted, and their numbers on each replica were averaged. Colonies of P. haemolytica and P. multocida were identified by colony morphology, Gram staining, and biochemical reactions and by use of specific antisera for serotyping P. haemolytica isolates (8, 9, 34).
Preparation of clinical samples for virus isolation.
The nasal swab samples were thawed at 25°C, and 1 ml of cold Dulbecco's modified minimum essential medium (DMEM) was added to each tube. Aliquots of these fluids were centrifuged at 2,000 × g (model TJ-6R; Beckman Instruments, Inc., Palo Alto, Calif.) for 20 min. Supernatant fluids were withdrawn and filtered through Millipore filters (Gelman Sciences, Ann Arbor, Mich.) with a pore size of 0.450 μm. The filtrates were then used to inoculate the cell cultures. Lung tissues were minced and homogenized by grinding with Ten Broeck devices in the appropriate volume of cold DMEM to make 5% (wt/vol) suspensions. The lung homogenates were centrifuged at 2,000 × g. The top half of the supernatant was withdrawn, diluted 1:1 with cold DMEM, and subjected to a 2nd cycle of centrifugation. The top half of this supernatant was withdrawn, filtered as indicated, and used for virus isolation tests or RBCV infectivity titration (28, 30).
Virus isolation methods.
Specific virus isolation tests depended on three different cell types selectively permissive for the known respiratory bovine viruses. Cell cultures included the G clone of human rectal tumor-18 (HRT-18) cells specifically permissive for RBCV (19, 28, 33), Georgia bovine kidney (GBK) cells permissive for BHV-1, PI-3, and bovine adenoviruses (BAV), as well as bovine turbinate (BT) cells highly permissive for BRSV and cytopathogenic BVDV, and, to a lesser degree, permissive for BHV-1, PI-3, and BAV (28). These cell types were propagated in 24-well cluster plates (Becton-Dickinson Labware, Franklin Lakes, N.J.) and used in virus isolation tests by inoculating four wells: two of them with 10−1 and two with 10−2 dilutions of each test sample. Cells in four wells remained as uninoculated cell controls for each test plate. The inoculated cell cultures were incubated at 37°C and examined for cytopathic changes with an inverted microscope for 2 to 5 days. The cell cultures then were frozen at −70°C and thawed at 25°C. Cultures with cytopathic changes after inoculation with specific samples were pooled, and those remaining normal were also pooled as single samples for subpassages in the respective permissive cell cultures and for detecting hemagglutinin (HA) or receptor-destroying enzyme (RDE) activities (28, 31).
Identification of virus isolates.
The virus isolates were initially differentiated by characteristics of cytopathic effects in the specifically permissive cell types. Additional virus identification tests included HA detection with rat erythrocytes (RBC) and assays for RDE functions mediated by acetylesterase (AE), a characteristic of RBCV. Tests for HA of PI-3 involved the use of bovine and chicken RBC. Virus isolates were further identified by using specific antisera in HA inhibition (HAI), infectivity neutralization tests, or negative-contrast staining and electron microscopic examinations (28).
Quantification of RBCV in the infected lung tissues.
The plaque test was used to assess the titers of PFU of RBCV in 25 available lung samples according to recently described procedures (19). The lung samples prepared for virus isolations were serially diluted in 10-fold steps, and 0.5-ml aliquots of the respective dilutions were placed on confluent G clone cell monolayers grown in six-well cluster plates. (Becton-Dickinson Labware, Franklin Lakes, N.J.). The plaque test for PI-3 infectivity involved GBK cell cultures and was otherwise identical. The plaque test for BHV-1 employed GBK cell cultures, a 2% methylcellulose overlay, and crystal violet staining.
Tests for viral HA and RDE.
Washed rat RBC at a concentration of 0.5% in PBS at pH 7.4 containing 0.05% bovine serum albumin (BSA) were used to detect HA antigens of RBCV in infected G clone cultures. The same concentrations of bovine or chicken RBC were used to detect HA of PI-3 isolates. Twofold dilutions of 50 μl of the samples were made in 96-well V-bottom microtiter plates (Becton Dickinson Labware, Franklin Lakes, N.J.), and equal volumes of RBC suspensions were added to each well (31). The plates were shaken and incubated at 6°C for 2 h, which is sufficient time for the RBC to form clear buttons in diluent control wells. The HA titers were recorded as the highest dilution with complete agglutination of RBC suspensions. The plates were then incubated at 37°C for activation of the RDE function. The RDE titer was determined by elution of virus from rat RBC in wells with previous agglutination and was recorded after 2 h as the highest dilution with settled RBC (31).
HAI tests.
Sequentially serum samples from the 26 cattle with fatal pneumonia and from 18 normal control cattle were tested for HAI antibodies against RBCV antigen. These sera were also tested for antibodies against P. haemolytica antigen in an enzyme-linked immunosorbent assay (ELISA). The serum samples were diluted 1:4 in PBS containing 0.05% BSA, heat-inactivated at 56°C for 30 min, and then diluted in twofold steps in 50-μl aliquots. An antigen extracted from RBCV-infected cell lysates was used and diluted to contain 8 to 16 U of HA and RDE. The antigen was added in 50-μl volumes to each serum dilution. The serum-antigen mixtures reacted for 30 min at 25°C, and 50 μl of rat RBC suspensions then was added. The test plates were kept at 6°C for 2 h, and the HAI titers were determined as the highest dilutions inhibiting HA of RBCV. Serum 1745 with a known HAI antibody titer and normal serum were included as positive and negative controls, respectively.
Determination of P. haemolytica antibodies.
A 32-kDa outer membrane protein of P. haemolytica was harvested from 18- to 24-h cultures in beef heart infusion broth as the supernatant after a 10,000 × g centrifugation. The antigen preparation was diluted 1:12.5, test sera were diluted 1:50, and the ELISA was performed as described previously (3).
RESULTS
Shedding of RBCV and Pasteurella spp. in nasal secretions before death.
Virus and bacterial isolation results from nasal swab samples of the 26 fatal cases from the two epizootics are recorded in Table 1. In the 1997 experiment, six cattle shed G clone-dependent RBCV in nasal secretions on day 0, and three additional calves contracted this infection on day 5. In the 1998 experiment, 15 of the 16 cattle shed RBCV at the beginning of the experiment, and all of them did so on day 5. Other viruses were not isolated from nasal discharges of these 26 cattle during the first 5 days of the experiments. Three cattle, surviving 14 to 36 days after onset of this epizootic, began shedding BHV-1 on day 12 and PI-3 on day 26 (data not shown). Calf 98TXSF-115 had a dual infection with RBCV and BHV-1 on day 12, and calves 98TXSF-15 and 98TXSF-100 nasally shed BHV-1 on days 12 and 19. Calf 98TXSF-15 nasally shed BHV-1 and PI-3 on day 26 (data not shown). Calf 98TXSF-100 was nasally BHV-1 isolation negative on day 26, and became PI-3 isolation positive on day 33 (data not shown). Uninoculated cell culture controls maintained normal morphological features in all of these virus isolation tests. Two of the 26 cattle shed P. haemolytica on day 0, and 17 of them carried P. haemolytica after transport on day 5. The 18 normal control cattle did not secrete RBCV in their nasal discharges during the test periods (30). None of the 18 normal control cattle had Pasteurella spp. in nasal swabs on day 0, and 3 of them nasally shed P. haemolytica on day 5 (data not shown).
TABLE 1.
Isolation of viruses and Pasteurella spp. from nasal secretions of cattle with fatal SFP in the 1997 and 1998 epizootics
| Calf | Day of death | Isolation of agent on daya: | |||||||
|---|---|---|---|---|---|---|---|---|---|
| RBCV | Pasteurella spp. | ||||||||
| 0 | 5 | 12 | 19 | 0 | 5 | 12 | 19 | ||
| 97TXSF-52 | 5 | − | + | − | − | ||||
| 97TXSF-5 | 6 | + | + | − | − | ||||
| 97TXSF-15 | 6 | + | + | − | − | ||||
| 97TXSF-19 | 6 | + | + | − | A1b | ||||
| 97TXSF-43 | 6 | + | + | − | − | ||||
| 97TXSF-62 | 6 | − | + | A1 | − | ||||
| 97TXSF-75 | 6 | − | + | − | A1 | ||||
| 97TXSF-96 | 6 | + | + | − | − | ||||
| 97TXSF-58 | 7 | + | + | − | − | ||||
| 97TXSF-63 | 9 | − | − | − | − | ||||
| 98TXSF-85 | 5 | − | + | − | A1 | ||||
| 98TXSF-10 | 6 | + | + | − | A1 | ||||
| 98TXSF-11 | 6 | + | + | − | A1 | ||||
| 98TXSF-14 | 6 | + | + | − | A1 | ||||
| 98TXSF-91 | 6 | + | + | − | A1 | ||||
| 98TXSF-110 | 6 | + | + | − | A1 | ||||
| 98TXSF-71 | 7 | + | + | − | A1 | ||||
| 98TXSF-72 | 7 | + | + | − | A1 | ||||
| 98TXSF-106 | 7 | + | + | − | A1 | ||||
| 98TXSF-114 | 7 | + | + | − | A1 | ||||
| 98TXSF-102 | 12 | + | + | − | A1 | ||||
| 98TXSF-104 | 13 | + | + | − | − | − | − | ||
| 98TXSF-115 | 14 | + | + | +/BHV-1 | − | A1 | A1 | ||
| 98TXSF-36 | 27 | + | + | − | − | − | A1 | − | − |
| 98TXSF-15 | 31 | + | + | −/BHV-1 | −/BHV-1 | − | A1 | − | − |
| 98TXSF-100 | 36 | + | + | −/BHV-1 | −/BHV-1 | A1 | A1 | − | − |
−, RBCV or Pasteurella spp. not isolated; +, RBCV isolated.
A1, P. haemolytica type A1.
Combined lung infections with RBCV and Pasteurella spp. and respective titers in the 1997 and 1998 epizootics.
Pneumonic lung samples from 9 of 10 cattle of the 1997 experiment, and from 9 of 16 cattle of the 1998 experiment were RBCV isolation positive when they died between days 5 and 7 (Table 2). The RBCV titers ranged from 1 × 103 to 1.2 × 107 PFU per g of lung tissues. BHV-1 was detected at 2 × 103 PFU per g of lung tissue of calf 98TXSF-115, which died on day 14. Less than 103 PFU of PI-3 per g of lung tissue were detected in calves 98TXSF15 and 98TXSF-100, which died on days 31 and 36, respectively. The last calf had the most drawn-out respiratory tract disease and was treated with antibiotics six times at different intervals. The lung of calf 98TXSF-114 was not available for virological testing. The 25 available lung tissues of 26 dead cattle tested did not yield BRSV, cytopathogenic BVDV, or BAV through inoculation of selectively permissive cell culture systems. Cells of the exfoliative or cryostat preparations from these lungs did not react with BVDV-specific fluorescent antibodies. This test was included to detect the potential presence of noncytocidal BVDV in these lungs.
TABLE 2.
Titers of viruses and Pasteurella spp. in lungs of cattle with fatal SFP in the 1997 and 1998 epizootics
| Calf | Day of death | Titer ofa: | |
|---|---|---|---|
| Virus (PFU/g) | Pasteurella spp. (CFU/g) | ||
| 97TXSF-52 | 5 | RBCV, 5.0 × 106 | A1, 1.0 × 106; P. multocida, 1.6 × 107 |
| 97TXSF-5 | 6 | RBCV, 1.2 × 105 | A1, 7.0 × 108 |
| 97TXSF-15 | 6 | RBCV, 3.0 × 106 | A6, 4.8 × 108 |
| 97TXSF-19 | 6 | RBCV, 8.0 × 104 | A1, 1.4 × 109 |
| 97TXSF-43 | 6 | RBCV, 8.0 × 103 | A1, 3.4 × 108 |
| 97TXSF-62 | 6 | RBCV, 1.0 × 105 | A1, 8.2 × 108 |
| 97TXSF-75 | 6 | RBCV, 1.4 × 106 | A1, 1.5 × 107 |
| 97TXSF-96 | 6 | RBCV, 3.4 × 106 | A1, 1.1 × 107 |
| 97TXSF-58 | 7 | RBCV, 2.0 × 105 | A1, 1.9 × 106 |
| 97TXSF-63 | 9 | A1, 2.8 × 106 | |
| 98TXSF-85 | 5 | RBCV, 6.0 × 104 | P. multocida, 6.0 × 105 |
| 98TXSF-10 | 6 | RBCV, 1.0 × 105 | A1, 3.3 × 108; P. multocida, 2.5 × 106 |
| 98TXSF-11 | 6 | RBCV, 6.0 × 106 | A1, 6.3 × 108 |
| 98TXSF-14 | 6 | RBCV, 1.0 × 103 | P. multocida, 2.4 × 108 |
| 98TXSF-91 | 6 | RBCV, 1.2 × 107 | A1, 4.0 × 107; P. multocida, 1.4 × 108 |
| 98TXSF-110 | 6 | RBCV, 2.4 × 106 | P. multocida, 2.3 × 109 |
| 98TXSF-71 | 7 | RBCV, 4.0 × 105 | A1, 3.0 × 108; P. multocida, 4.1 × 108 |
| 98TXSF-72 | 7 | RBCV, 2.0 × 104 | A1, 3.1 × 108; P. multocida, 2.1 × 108 |
| 98TXSF-106 | 7 | RBCV, 2.0 × 104 | A1, 2.5 × 106 |
| 98TXSF-114 | 7 | NAb | A1, 8.0 × 106 |
| 98TXSF-102 | 12 | A2, 9.5 × 105 | |
| 98TXSF-104 | 13 | A1, 2.9 × 106 | |
| 98TXSF-115 | 14 | BHV-1, 2.0 × 103 | A1, 4.0 × 105 |
| 98TXSF-36 | 27 | P. multocida, 1.1 × 108 | |
| 98TXSF-15 | 31 | PI-3, <1.0 × 103 | |
| 98TXSF-100 | 36 | PI-3, <1.0 × 103 | P. multocida, 7.5 × 107 |
A1, -2, and -6, P. haemolytica types A1, -2, and -6.
NA, not available.
P. haemolytica was cultured from the lungs of all 10 calves which died in the 1997 epizootic, as indicated in Table 2. Calf 97TXSF-15 had a pure culture of P. haemolytica A6. The lungs of the other nine calves had P. haemolytica A1 infections, and calf 97TXSF-52 also had P. multocida infections. Pasteurella spp. were isolated from 15 lungs of 16 calves which died in the 1998 epizootic (Table 2). Four calves (98TXSF-10, -71, -72, and -91) had mixed infections of P. haemolytica A1 and P. multocida. Calves 98TXSF-14, -36, -85, -100, and -110 had pure P. multocida infections of the lungs. The bacterial isolate from the lung of calf 98TXSF-102 was identified as a pure culture of P. haemolytica A2, while the bacterial isolates from the remaining five calves were identified as pure P. haemolytica A1. The lungs of calf 98TXSF-15 that died at the end of the study after repeated treatment with antibiotics were negative in bacterial cultures. The CFU of P. haemolytica varied from 4 × 105 to 1.4 × 109 per g of lung tissue. The CFU of P. multocida ranged from 6.0 × 105 to 2.3 × 109 per g of lung tissue.
Biological properties of the virus isolates.
The RBCV isolated from the pneumonic lungs were indistinguishable from the isolates detected in sequential nasal swab samples. These RBCV isolates exhibited high fusogenic functions in G clone cells in the first passage, did not replicate in GBK or BT cells, and agglutinated rat but not bovine or chicken RBC. All RBCV isolates destroyed rat RBC glycoconjugate receptors as a function of their RDE. Uniformly large RBCV plaque phenotypes with diameters of 5 to 6 mm were detected in the plaque titration of the pneumonic lungs of cattle in the 1997 and 1998 epizootics (data not shown). The viruses isolated by inoculation of GBK or BT cell cultures were identified as BHV-1 through their cytopathic changes consisting of clusters of rounded cells and neutralization by BHV-1-specific antiserum. They were not temperature sensitive like some commercial live BHV-1 vaccines because they multiplied at 36 and 39°C. The virus isolates from the lungs of calves 98TXSF-15 and 98TXSF-100 induced cell fusion in GBK or BT cells and agglutinated chicken and bovine RBC, and their HA was inhibited by PI-3-specific antiserum. These properties identified these isolates as PI-3.
Antibody responses to infections with RBCV and P. haemolytica of cattle developing fatal pneumonia and healthy resistant cattle.
The dead cattle of both epizootics had HAI antibody titers of <8 to 16 on day 0, with the exception of calf 97TXSF-63. These titers remained at these low levels during the first 5 days (Table 3). The HAI antibody titers of calf 97TXSF-63 that died on day 9 was 32 on day 0 and increased to 256 on day 5. Both nasal swab and lung samples from this calf remained negative for virus isolation. The three cattle of the 1998 experiment that died between days 27 and 36 had increasing HAI titers reaching 64. Isolation of RBCV from lung tissues of these cattle was unsuccessful. The ELISA results indicated that all except one calf were antibody negative for P. haemolytica antigen on day 0, and the antibody levels rose among 10 of 18 cattle that were tested on day 5 (Table 3). The four cattle that died between days 14 and 36 maintained such titers.
TABLE 3.
Antibody responses to RBCV and P. haemolytica of cattle with fatal SFP in the 1997 and 1998 epizootics
| Calf | Day of death | HAI titer to RBCV on daya: | Antibody titer to P. haemolytica on dayb: | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 5 | 12 | 19 | 0 | 5 | 12 | 19 | ||
| 97TXSF-52 | 5 | 8 | 8 | 0 | 0 | ||||
| 97TXSF-5 | 6 | 8 | 16 | 0 | 184 | ||||
| 97TXSF-15 | 6 | 16 | NAc | 0 | NA | ||||
| 97TXSF-19 | 6 | 16 | NA | 0 | NA | ||||
| 97TXSF-43 | 6 | 8 | NA | 0 | NA | ||||
| 97TXSF-62 | 6 | 8 | 16 | 0 | 0 | ||||
| 97TXSF-75 | 6 | 16 | 16 | 0 | 491 | ||||
| 97TXSF-96 | 6 | 16 | 16 | 0 | 0 | ||||
| 97TXSF-58 | 7 | 16 | 16 | 0 | 0 | ||||
| 97TXSF-63 | 9 | 32 | 256 | 0 | 445 | ||||
| 98TXSF-85 | 5 | 8 | NA | 0 | NA | ||||
| 98TXSF-10 | 6 | <8 | NA | 0 | NA | ||||
| 98TXSF-11 | 6 | 8 | NA | 0 | NA | ||||
| 98TXSF-14 | 6 | 8 | 8 | 0 | 0 | ||||
| 98TXSF-91 | 6 | 8 | NA | 0 | NA | ||||
| 98TXSF-110 | 6 | 8 | NA | 0 | NA | ||||
| 98TXSF-71 | 7 | 8 | 16 | 0 | 583 | ||||
| 98TXSF-72 | 7 | 8 | 16 | 48 | 253 | ||||
| 98TXSF-106 | 7 | <8 | 8 | 0 | 0 | ||||
| 98TXSF-114 | 7 | <8 | 8 | 0 | 0 | ||||
| 98TXSF-102 | 12 | <8 | 16 | 0 | 105 | ||||
| 98TXSF-104 | 13 | <8 | <8 | NA | 396 | 399 | NA | ||
| 98TXSF-115 | 14 | 8 | 8 | 8 | 0 | 299 | 442 | ||
| 98TXSF-36 | 27 | 16 | 16 | 64 | 64 | 0 | 522 | 396 | 173 |
| 98TXSF-15 | 31 | 8 | 16 | 32 | 64 | 0 | 563 | 698 | 668 |
| 98TXSF-100 | 36 | <8 | 8 | 32 | 64 | 0 | 0 | 593 | 102 |
An 8 indicates a titer with complete inhibition of RBCV HA.
A 0 indicates no antibody reaction in ELISA.
NA, not available.
The HAI antibody titers of the 18 normal control cattle that remained clinically healthy and did not nasally shed RBCV in the 1997 and 1998 experiments ranged from 16 to 1,024 on day 0 (Table 4). Their antibody titers continued to rise and remained high on days 12 and 19. Ten of the 18 cattle were antibody negative for P. haemolytica antigen on day 0, 16 had positive titers on day 5, and all had significant titers on days 12 and 19.
TABLE 4.
Antibody responses to RBCV and Pasteurella spp. of resistant cattle in the 1997 and 1998 epizootics of SFP
| Cow | HAI titer to RBCV on daya: | Antibody titer to P. haemolytica on dayb: | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 5 | 12 | 19 | 0 | 5 | 12 | 19 | |
| 97TXSF-2 | 256 | 256 | 256 | 128 | 640 | 549 | 897 | 708 |
| 97TXSF-4 | 16 | 512 | 1,024 | 256 | 0 | 606 | 904 | 844 |
| 97TXSF-9 | 32 | 512 | 512 | 256 | 0 | 716 | 674 | 843 |
| 97TXSF-29 | 16 | 256 | 512 | 256 | 315 | 485 | 599 | 967 |
| 97TXSF-32 | 64 | 512 | 512 | 256 | 0 | 418 | 675 | 609 |
| 97TXSF-41 | 128 | 256 | 512 | 256 | 0 | 702 | 818 | 702 |
| 97TXSF-55 | 64 | 256 | 512 | 512 | 177 | 330 | 463 | 679 |
| 98TXSF-2 | 16 | 16 | 64 | 256 | 705 | 516 | 690 | 572 |
| 98TXSF-27 | 128 | 256 | 256 | 256 | 0 | 607 | 834 | 710 |
| 98TXSF-30 | 64 | 1,024 | 1,024 | 1,024 | 0 | 0 | 613 | 532 |
| 98TXSF-32 | 128 | 128 | 128 | 128 | 0 | 532 | 762 | 833 |
| 98TXSF-34 | 512 | 512 | 512 | 256 | 54 | 115 | 338 | 251 |
| 98TXSF-44 | 16 | 64 | 512 | 256 | 0 | 0 | 103 | 519 |
| 98TXSF-47 | 256 | 256 | 1,024 | 1,024 | 478 | 447 | 466 | 429 |
| 98TXSF-52 | 64 | 256 | 256 | 512 | 462 | 504 | 464 | 465 |
| 98TXSF-93 | 1,024 | 1,024 | 256 | 256 | 0 | 487 | 492 | 515 |
| 98TXSF-94 | 256 | 256 | 256 | 512 | 0 | 170 | 625 | 636 |
| 98TXSF-119 | 128 | 128 | 128 | 128 | 452 | 732 | 625 | 708 |
A 256 indicates a titer with complete inhibition of RBCV HA.
A 0 indicates no antibody reaction in ELISA.
Clinical and pathological findings.
Ten of 105 cattle in the 1997 experiment died on days 5 to 9, and 16 of 120 cattle in the 1998 epizootic died on days 5 to 36 (Tables 1 and 2). They all developed fever as high as 44°C and severe signs of respiratory distress. The necropsy examinations revealed that 50 to 80% of the total lung volumes had subacute exudative or subacute exudative and necrotizing lobar pneumonia primarily affecting the anteroventral areas of the lungs. The exudative component of the pneumonic process consisted of fibrin deposited to various degrees on the pleural surfaces of lungs or within distended intralobular septae and airways. Gross lesions in the gastrointestinal tracts or other organ systems of these cattle were not detected. All cattle were treated under good nutritional conditions.
Histological changes.
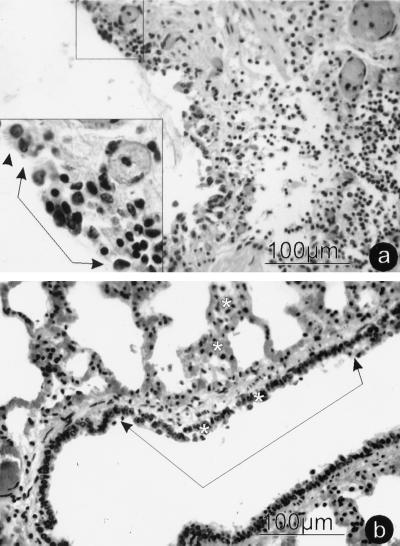
The microscopic changes in a majority of the pneumonic lung lobules were characterized as acute and subacute fibrinous bronchopneumonia. Typical exudative lesions associated with pneumonic pasteurellosis were not evident in isolated lung lobules. These lobules had moderate to severe bronchitis and bronchiolitis consisting of degenerating and necrotic respiratory epithelium with subepithelial and intraepithelial, nonsuppurative inflammatory cell infiltration (Fig. 1). Hyperchromatic and fused epithelial cells were present in airway epithelium adjacent to areas of necrosis, degeneration, and intraepithelial inflammation. These changes were detected at all levels of the lower respiratory tract from small bronchi to alveolar ducts. Many alveoli were no longer lined by type I pneumocytes and were partially filled with pink, protein-rich exudates and mixtures of small and large mononuclear leukocytes. Intracytoplasmic or intranuclear inclusions were not discernable. Cytoplasmic fluorescence was detected in epithelial cells of bronchi, broncheoli, and alveolar ducts when cryostat sections of the lungs of cattle that died within 7 days were tested with fluorescing antibodies against RBCV (data not shown). Cells of these lung sections did not fluoresce when treated with RBCV antibody-negative serum or with BVDV antiserum.
FIG. 1.
Photomicrographs of initial histological changes in lung from calf 98TXSF-11. (a) Small bronchus revealing respiratory epithelium degeneration and necrosis with subepithelial and intraepithelial mononuclear inflammatory infiltrates. (Inset) A single arrowhead identifies the margin of normal ciliated epithelium; connected arrowheads mark epithelial degeneration and necrosis with intraepithelial inflammation. The inset is a magnification of the area boxed at the top of the panel. (b) Respiratory bronchiole and alveolar duct demonstrating epithelial cell degeneration and necrosis. Asterisks identify areas of mononuclear cell infiltration in and adjacent to respiratory epithelium and alveolar walls. The infectious load in this lung was 6 × 106 PFU of RBCV per g and 6.3 × 108 CFU of P. haemolytica A1 per g.
DISCUSSION
The prospective experimental designs of our investigations permitted sequential tracing of shedding of viruses and bacteria in nasal discharges during the naturally occurring course of fatal SFP pathogenesis. Virtual experimental induction of SFP thus was monitored through clinical, virological, bacteriological, serological, and pathological evaluations. Epizootics of SFP had not been explored in the past by such approaches (37). Significantly, 25 of 26 calves that died profusely shed RBCV in nasal secretions during the early phases of the two epizootics of SFP. In addition, this previously not recognized RBCV infection was present in the lungs of all cattle that died between days 5 and 7 of the experiments. However, RBCV was not isolated from the lungs of calves dying on days 9 to 36, most of which had significant HAI antibody titers when they died. Large RBCV plaque phenotypes with titers reaching 1.2 × 107 PFU per g of tissue were detected in the lungs. Simultaneously, the lungs from 25 of 26 cattle had infections with Pasteurella spp. ranging in titers between 4 × 105 and 2.3 × 109 CFU per g of lung tissue. Importantly, shedding of these bacteria in nasal secretions was low at the beginning of the experiments when RBCV was shed abundantly in nasal discharge. Clinically normal control cattle or cattle with chronic lung worm infections did not shed RBCV in this and other related investigations (29, 30).
Histological changes consisted of lung lobules typical of virus infections and lung lobules with fibronecrotic pneumonia. Similar sequential infectious interplays were reported in human subjects suffering from dual infections with influenza virus of type A and Staphylococcus, Haemophilus, or Streptococcus spp. and in experimentally induced, combined virus and bacterial infections of mice, swine, and cattle (15, 16). The upper respiratory tracts but not lungs of clinically normal cattle may harbor Pasteurella spp. and other bacteria (9–11, 14, 22). Bacterial invasion and multiplication in the lungs are associated with pathogenic processes of SFP (6, 14, 35, 37). These data substantiate our hypothesis that RBCV infections are causatively associated with these epizootics of SFP.
The RBCV isolated from nasal swab and lung samples expressed RDE functions mediated by an AE that hydrolyzes an ester bond to liberate acetate from sialic acid-containing bovine submaxillary mucin, a substance with a chemical composition resembling the glycocalyx that covers the bovine respiratory tracts (12, 31). Pathogenetic mechanisms probably involved action of this viral enzyme by inducing glycocalyx changes that lowered mucosal resistance barriers and favored virus penetration and adhesion of P. haemolytica or P. multocida to cells of the lower respiratory tracts.
Our refined virus isolation tests permitted the exclusion of other respiratory bovine viruses that could have infected the cattle during the initial stages of SFP pathogenesis (28, 30). Immunofluorescence tests for noncytocidal BVDV infections were negative when lung samples were tested with BVDV-specific antibodies. Cytopathogenic BVDV, BRSV, or BAV were not detected in nasal secretions or lungs through virus isolation attempts with GBK and BT cell cultures (28). Three calves that died on days 14, 31, and 36 with protracted pneumonia nasally shed BHV-1 or PI-3 late in the 1998 epizootic. All of these cattle had initial respiratory tract infections with RBCV on days 0 and 5 and had been given BHV-1 vaccines.
Numerous past attempts at inducing SFP have not reproduced the naturally occurring disease with shipping stress alone; with experimental aerosol inoculation of BHV-1, PI-3, or BRSV alone; or with bacterial aerosol exposure alone, unless large quantities of P. haemolytica organisms were introduced directly into the lungs (5, 11, 24, 36, 37). Investigations of sequential infections with these viruses and P. haemolytica were also reported (2, 18, 25, 27, 37). More severe clinical signs were observed in experiments with combined infections than with single infections. The loads of the infectious components in the lungs of experimental cattle were not assessed in any of these investigations. While these types of experiments induced respiratory tract diseases, they did not have the typical features of naturally occurring SFP. The concept of SFP resulting from the interplay of multifactorial burdens on respiratory health of cattle is widely accepted (13, 32, 37). Stress readily is generated at weaning, in auction markets, during transport, and with adjustment to the feed yard environments. These conditions also facilitate rapid spread of viral and bacterial infections.
The etiological roles of infectious agents and their mechanisms of pathogenesis in SFP have been researched in numerous past investigations, but they must still be further defined. The original Henle-Koch's postulates have not been proven for a disease as complex as SFP (32, 37). Evans analyzed similar challenges involving the roles of viruses in the genesis of chronic diseases, several forms of cancer, or other complex human disease conditions (7). He formulated a unified concept of criteria for causation in order to identify specific etiological factors in the genesis of complex and chronic diseases. Thomson first related these criteria for causation to infections leading to SFP (32).
Criteria for the involvement of different infectious factors in SFP were evaluated by us according to Thomson's ideas about Evans' criteria (7, 32). The potential etiological roles of RBCV or other respiratory bovine viruses as causative factors in the pathogenesis of the 1997 and 1998 epizootics of SFP were applied to the following criteria. (i) The virus infects the mucosa of respiratory tract passages and lungs of affected cattle. (ii) The virus can be isolated in cell cultures at high rates from respiratory secretions and lung samples during the pathogenesis of SFP. Both of these criteria were proven by the results of investigations described in this and related reports (28, 30). (iii) Virus-specific immune responses are observed in cattle that recover from SFP. Rising titers of HAI antibodies against RBCV were detected in all surviving calves which had RBCV infections on days 0, 5, and later (data not shown). They developed typical primary antibody responses to RBCV infections characterized by increases in immunoglobulin M (IgM) appearing first, followed by rises in IgG1 and IgG2 (20, 21, 30). In contrast, the RBCV isolation-positive cattle with fatal outcomes had no or low titers of HAI antibodies against RBCV in the early stages of the epizootics. These cattle developed only initial IgM responses to RBCV infections before they died (20, 21). (iv) Viruses isolated from cattle with SFP are not isolated from clinically normal cattle, but they may be detected in the pathogenesis of other respiratory tract diseases (1, 29). Besides the 18 normal control cattle of this report, 20 normal cattle and 32 cattle with chronic lungworm infections did not shed RBCV in nasal secretions in related investigations (29, 30). (v) Cattle with significant antibody titers against the candidate virus do not develop SFP, which occurs in cattle without such immune protection. Eighteen normal control cattle remaining clinically healthy and RBCV isolation negative (7 in 1997 and 11 in 1998) had significant titers of HAI antibodies against RBCV at the beginning of the epizootics. These cattle had the highest levels of total and IgG2 antibodies against RBCV (20, 21). In contrast, calves without such immune protections developed acute respiratory tract disease, including the fatal cases described above. (vi) Elimination of the virus factor prevents or decreases the severity of SFP. This criterion awaits the development of an effective vaccine. (vii) “The whole thing should make biologic and epidemiologic sense” (7, 32). The virological, bacteriological, immunological, epidemiological, pathological, and histological findings on cattle of these two experimentally monitored epizootics of SFP satisfy this criterion to a full measure.
Application of these criteria to virus infections of the two epizootics identifies RBCV in these fatal cases of SFP as an initiating and significant infection that was previously not recognized. This report describes for the first time initial high rates of respiratory tract infections with a virus and the evolving secondary infections with Pasteurella spp. among cattle developing fatal SFP under experimentally controlled conditions in natural settings of two severe epizootics.
The initial high rates of nasal RBCV shedding followed by lung infections with RBCV and P. haemolytica or P. multocida were proven through cultivation and quantification of these infectious agents in nasal secretions and affected lungs. The fatal outcomes of the combined infections of lungs with RBCV and Pasteurella spp. probably were influenced by the bacterial component which induced necrotizing lesions in the lungs. Importantly, normal control cattle that remained healthy and were refractile to infectious insults entered the evolving epizootics with significant HAI antibody titers to RBCV, and they mounted antibody responses to antigens of P. haemolytica within 5 days.
ACKNOWLEDGMENTS
This work was supported by grants from the Critical Issues and the National Research Initiative Programs of the United States Department of Agriculture (98-35204-6585, 98-34362-6071, and 94-37204-0926); Texas Agricultural Experiment Station Project H-3074 (Regional Research NC107); Texas Advanced Technology Program (grant no. 999902); the Louisiana Education Quality Support Fund (RF/1995-1998 RD-B-18) with matches from Immtech Biologics, Inc., Bucyrus, Kans., and Bayer Corporation, Merriam, Kans.; the Louisiana Agricultural Experiment Station; the Louisiana Beef Industry Council; and the School of Veterinary Medicine, Louisiana State University, Baton Rouge, La.
We thank Richard E. Corstvet for ELISA analysis of antibody responses against P. haemolytica and William G. Henk for digital processing of Fig. 1.
REFERENCES
- 1.Appel G, Heckert H-P, Hofmann W. Über die Beteiligung von bovinem Coronavirus (BCV) am Rindergrippekomplex in Betrieben Schleswig-Holsteins. Tieraerztl Umsch. 1992;47:296–304. [Google Scholar]
- 2.Baldwin D A, Marshall R G, Wessman G E. Experimental infection of calves with myxovirus parainfluenza-3 and Pasteurella haemolytica. Am J Vet Res. 1967;28:1773–1782. [PubMed] [Google Scholar]
- 3.Brennan R E, Corstvet R E, Paulson D B. Antibody responses to Pasteurella haemolytica 1:A and three of its outer membrane proteins in serum, nasal secretions, and bronchoalveolar lavage fluid from calves. Am J Vet Res. 1998;59:727–732. [PubMed] [Google Scholar]
- 4.Briggs R E, Frank G H, Purdy C W, Zehr E S, Loan R W. Rapid spread of a unique strain of Pasteurella haemolytica serotype 1 among transported calves. Am J Vet Res. 1998;59:401–405. [PubMed] [Google Scholar]
- 5.Ciszewski D K, Baker J C, Slocombe R F, Reindel J F, Haines D M, Clark E G. Experimental reproduction of respiratory tract disease with bovine respiratory syncytial virus. Vet Microbiol. 1991;28:39–60. doi: 10.1016/0378-1135(91)90098-z. [DOI] [PubMed] [Google Scholar]
- 6.DeRosa D C, Mechor G D, Staats J J, Chengappa M M, Shryock T R. Comparison of Pasteurella spp. simultaneously isolated from nasal and transtracheal swabs from cattle with clinical signs of respiratory disease. J Clin Microbiol. 2000;38:327–332. doi: 10.1128/jcm.38.1.327-332.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evans A S. Causation and disease: the Henle-Koch postulates revisited. Yale J Biol Med. 1976;49:175–195. [PMC free article] [PubMed] [Google Scholar]
- 8.Frank G H, Wessman G E. Rapid plate agglutination procedure for serotyping. J Clin Microbiol. 1978;7:142–145. doi: 10.1128/jcm.7.2.142-145.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frank G H, Smith P C. Prevalence of Pasteurella haemolytica in transported calves. Am J Vet Res. 1983;44:981–985. [PubMed] [Google Scholar]
- 10.Frank G H, Briggs R E. Colonization of the tonsils of calves with Pasteurella haemolytica. Am J Vet Res. 1992;53:481–484. [PubMed] [Google Scholar]
- 11.Friend S C E, Thomson R G, Wilkie B N. Pulmonary lesions induced by Pasteurella haemolytica in cattle. Can J Comp Med. 1977;41:212–223. [PMC free article] [PubMed] [Google Scholar]
- 12.Herrler G, Rott R, Klenk H D, Müller H P, Shukla A K, Schauer R. The receptor-destroying enzyme of influenza C virus: neuraminidate-O-acetyl-esterase. EMBO J. 1985;4:1503–1506. doi: 10.1002/j.1460-2075.1985.tb03809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoerlein A B. Shipping fever. In: Amstutz H E, editor. Bovine medicine and surgery. Santa Barbara, Calif: American Veterinary Publications, Inc.; 1980. pp. 99–106. [Google Scholar]
- 14.Hoerlein A B, Saxena S P, Mansfield M E. Studies on shipping fever of cattle. II. Prevalence of Pasteurella species in nasal secretions from normal calves and calves with shipping fever. Am J Vet Res. 1961;22:470–472. [PubMed] [Google Scholar]
- 15.Jakab G J. Mechanisms of virus-induced bacterial superinfection on the lung. Clin Chest Med. 1981;2:59–66. [PubMed] [Google Scholar]
- 16.Jakab G J. Viral-bacterial interaction in pulmonary infections. Adv Vet Sci Comp Med. 1982;26:155–171. [PubMed] [Google Scholar]
- 17.Jensen R, Pierson R E, Brady P M, Saari D M, Lauerman L H, England J J, Keyvanfar H, Collier J R, Horton D P, McChesney A E, Benitez A, Christie R M. Shipping fever pneumonia in yearling feedlot cattle. J Am Vet Med Assoc. 1976;169:500–506. [PubMed] [Google Scholar]
- 18.Jericho K W F, Langford E V. Pneumonia in calves produced with aerosols of bovine herpesvirus 1 and Pasteurella haemolytica. Can J Comp Med. 1978;42:269–277. [PMC free article] [PubMed] [Google Scholar]
- 19.Lin X Q, O'Reilly K L, Storz J. Infection of polarized epithelial cells with enteric and respiratory tract bovine coronaviruses and release of virus progeny. Am J Vet Res. 1997;58:1120–1124. [PubMed] [Google Scholar]
- 20.Lin, X. Q., K. L. O'Reilly, J. Storz, C. W. Purdy, and R. W. Loan. Antibody responses to respiratory coronavirus infections of cattle during shipping fever pathogenesis. Arch. Virol., in press. [DOI] [PMC free article] [PubMed]
- 21.Lin X Q. Isolation and characterization of newly emerging coronaviruses in acute respiratory tract diseases of cattle. Ph.D. dissertation. Baton Rouge, La: Louisiana State University; 2000. [Google Scholar]
- 22.Magwood S E, Barnum D A, Thomson R G. Nasal bacterial flora of calves in healthy and in pneumonia-prone herds. Can J Comp Med. 1969;33:237–243. [PMC free article] [PubMed] [Google Scholar]
- 23.McKercher D G, Moulton J E, Madin S H, Kendrick J W. Infectious bovine rhinotracheitis—a newly recognized virus disease of cattle. Am J Vet Res. 1957;18:246–256. [PubMed] [Google Scholar]
- 24.McKercher D G, Wada E M, Straub O C. Distribution and persistence of infectious bovine rhinotracheitis virus in experimentally infected cattle. Am J Vet Res. 1963;24:510–514. [PubMed] [Google Scholar]
- 25.Potgieter L N D, McCracken M D, Hopkins F M, Walker R D, Gay J S. Experimental production of bovine respiratory tract disease with bovine viral diarrhea virus. Am J Vet Res. 1984;45:1582–1585. [PubMed] [Google Scholar]
- 26.Reisinger R C, Heddleston K L, Manthei C A. A myxovirus (SF-4) associated with shipping fever of cattle. J Am Vet Med Assoc. 1959;135:147–154. [PubMed] [Google Scholar]
- 27.Saunders J R, Berman D T. Epizootiological studies of shipping fever II. Exposure of calves to pasteurellae and parainfluenza 3 virus. Can J Comp Med. 1964;28:47–62. [PMC free article] [PubMed] [Google Scholar]
- 28.Storz J, Stine L, Liem A, Anderson G A. Coronavirus isolation from nasal swab samples of cattle with signs of respiratory tract disease after shipping. J Am Vet Med Assoc. 1996;208:1452–1456. [PubMed] [Google Scholar]
- 29.Storz J. Respiratory disease of cattle associated with coronavirus infections. In: Howard J L, Smith R A, editors. Current veterinary therapy: food animal practice. 4th ed. Philadelphia, Pa: W. B. Saunders Co.; 1998. pp. 291–293. [Google Scholar]
- 30.Storz J, Purdy C W, Lin X Q, Burrell M, Truax R E, Briggs R E, Loan R W. Isolation of respiratory coronaviruses, other cytocidal viruses and Pasteurella spp from cattle involved in two natural outbreaks of shipping fever. J Am Vet Med Assoc. 2000;216:1599–1604. doi: 10.2460/javma.2000.216.1599. [DOI] [PubMed] [Google Scholar]
- 31.Storz J, Zhang X M, Rott R. Comparison of hemagglutinating, receptor-destroying, and acetylesterase activities of avirulent and virulent bovine coronavirus strains. Arch Virol. 1992;125:193–204. doi: 10.1007/BF01309637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thomson, R. G. A perspective on respiratory disease of feedlot cattle. Can. Vet. J. 21:181–185. [PMC free article] [PubMed]
- 33.Tompkins W A T, Watrach A M, Schmale J D, Schultz R M, Harris J A. Cultural and antigenic properties of newly established cell strains derived from adenocarcinomas of the human colon and rectum. J Natl Cancer Inst. 1974;52:904–911. doi: 10.1093/jnci/52.4.1101. [DOI] [PubMed] [Google Scholar]
- 34.Weaver R E, Hollis D G. Gram-negative fermentative bacteria and Francisella tularensis. In: Lennette E H, Balows A, Hausler W J Jr, Truant J P, editors. Manual of clinical microbiology. 3rd ed. Washington, D.C.: American Society for Microbiology; 1980. pp. 242–262. [Google Scholar]
- 35.Whitely L O, Maheswaran S K, Weiss D J. Pasteurella haemolytica A1 and bovine respiratory disease: pathogenesis. J Vet Intern Med. 1992;6:11–22. doi: 10.1111/j.1939-1676.1992.tb00980.x. [DOI] [PubMed] [Google Scholar]
- 36.Woolums A R, Anderson M L, Gunther R A, Schlelegle E S, La Rochelle D R, Singer R S, Boyle G A, Friebertshauser K E, Gershwin L J. Evaluation of severe disease induced by aerosol inoculation of calves with bovine respiratory syncytial virus. Am J Vet Res. 1999;60:473–480. [PubMed] [Google Scholar]
- 37.Yates W D G. A review of infectious bovine rhinotracheitis, shipping fever pneumonia and viral-bacterial synergism in respiratory disease of cattle. Can J Comp Med. 1982;46:225–263. [PMC free article] [PubMed] [Google Scholar]