Coronavirus disease 2019 (Covid-19) is a primarily respiratory tract infection caused by a newly recognised betacoronavirus named severe acute respiratory syndrome-related coronavirus (SARSr-CoV), first diagnosed in China (Wuhan), in December 2019.1
Since then, the outbreak has spread across the entire globe causing tens of thousands deaths, thus becoming a major public health emergency worldwide. Previous studies from China and the USA have reported overweight and obesity to be associated with a higher risk of severe pneumonia requiring invasive ventilation with intensive care unit (ICU) admission.2
However, no data exist about the relation between obesity and mortality in Covid-19 patients after ICU admission. In this retrospective study we analysed a cohort of consecutive patients with laboratory-confirmed COVID-19 infection treated with invasive ventilation and admitted to the ICU of Guglielmo da Saliceto Hospital in Piacenza (Italy) from February to April 2020.
Case data included demographics, clinical characteristics, laboratory findings and outcome (30-day mortality). Continuous data are reported as median (interquartile range; IQR). The Mann–Whitney test was used to compare the survivor and the non-survivor groups. Categorical variables were expressed as numbers (%) and compared by the χ2 test or Fisher’s exact test, as required.
Patients were divided according to the following body mass index (BMI) classes based on World Health Organization cut points: underweight (under 18.5 kg/m2); normal weight (18.5–25 kg/m2); overweight (25–30 kg/m2); obese class I (30–35 kg/m2); obese class II (35–40 kg/m2) and obese class III (>40 kg/m2).3
Univariable and multivariable logistic regression was used to assess the relationship between BMI and 30-day mortality. Results were expressed as odds ratios (ORs) with 95% confidence intervals (CIs) and P values. Statistical significance was set at a two-tailed P value of less than 0.05. Statistical analysis was performed using STATA V.16 (STATA Corp., TX, USA).
The study included 242 patients 80% of these patients have underlying clinical conditions, mainly arterial hypertension, diabetes and cardiovascular disease.
The median time from hospitalisation to ICU admission was 4 days (1–6 IQR) while the median time from ICU admission to death or discharge was 6 days (4–9 IQR).
Patients who died were older with a higher prevalence of chronic obstructive pulmonary disease (COPD) and lower arterial oxygen tension/fractional inspired oxygen ratio.
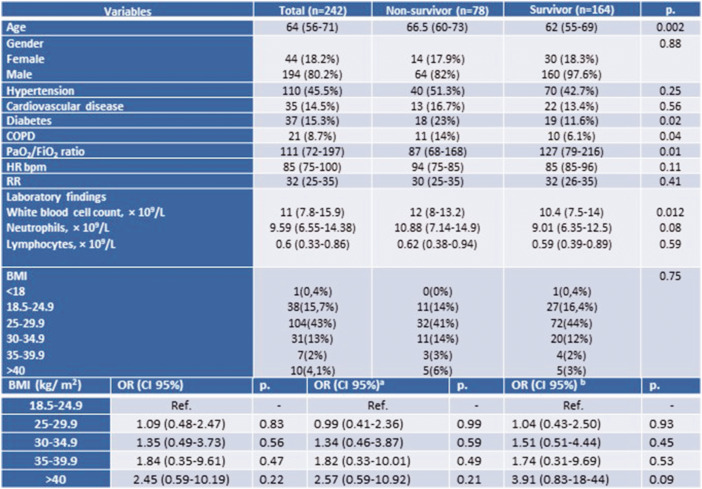
The median BMI in the overall population was 27.7 kg/m2 (25.4–29.7) and no differences in median BMI were observed between survivors and non-survivors (27.4 kg/m2 (25.4–29.4) vs. 27.9 kg/m2 (25.6–31.1), respectively, P = NS) (Figure 1).
Figure 1.
Study population characteristics and multivariable regression analysis.
Columns refer to overall study sample and compare the groups of survivors and non-survivors. P value refers to either Student’s t-test or χ2 test and the Fisher’s exact test for categorical variables. Data are n (%), n/N (%), mean and median (interquartile range; IQR). COPD: chronic obstructive pulmonary disease; RR: respiratory rate; HR: heart rate.
aOdds ratios are adjusted for gender and comorbidities (hypertension, cardiovascular disease, COPD and diabetes).
bOdds ratios are adjusted for age, gender and comorbidities (hypertension, cardiovascular disease, COPD and diabetes).
In both groups the majority of the patients presented with a BMI between 25 and 29.9, while five patients in the survivor group had a BMI greater than 40. The different BMI classes were not associated with an increased risk of 30-day mortality (crude OR) at univariable logistic regression. The same results were found after adjustment for age, gender and pre-existing medical conditions or for gender and pre-existing medical conditions (Figure 1). Severe obesity showed a significant association with higher 30-day mortality, also confirmed after excluding age from multivariate analysis.
To our knowledge, this research represents the first case series of sequentially hospitalised patients with confirmed Covid-19 and treated with invasive ventilation, exploring the BMI as an ominous prognostic factor in a Caucasian population. Our findings support the link between overweight and obesity and the risk of developing severe acute respiratory distress syndrome (ARDS) in patients with Covid-19, as the median BMI was 27.7 for those admitted to the ICU. There are several explanations for this finding: obesity is associated with impaired total lung capacity, functional residual capacity and vital capacity, and consequently with atelectasis, increased airway resistance and closure and ventilation/perfusion mismatch.4
We observed that only was morbid obesity associated with 30-day mortality, in keeping with some evidence demonstrating that despite obesity and overweight being associated with a greater risk of the development of ARDS/acute lung injury and difficulties of intubation, but once admitted to the ICU, the risk of death in obese and overweight patients was unchanged or even decreased.5
The protective role of higher BMI in advanced clinical conditions has been defined as ‘the obesity paradox’, and has also been observed in heart failure and cancer patients.6,7
This phenomenon has been associated with confounding factors, such as age or severity score. In a recent paper, obese patients with ARDS were systematically younger, with lower disease severity compared with normal weight patients.8 This was not the case in our study, in which at multivariate analysis, obesity was not associated with younger age.
Various pathophysiological mechanisms could explain ‘the obesity paradox’: a protective response called ‘preconditioning cloud’ in which obesity induces a low-grade inflammation that subsequently protects the lung against further insults; the high chest wall elastance could redistribute regional transpulmonary pressure, possibly reducing the potential negative effects of mechanical ventilation in an inhomogeneous lung.9 Some authors argued that the better prognosis of obese patients could be attributed to limitations of BMI as a synthetic anthropometric measure. To verify this hypothesis Aimo et al.10 were the first to estimate in heart failure patients the percentage of body weight composed of fat tissue (PBF) as a prognostic factor more closely correlated to body composition. The authors confirmed that heart failure patients with a BMI greater than 25 have a reduction of cardiovascular mortality and, most notably, patients with higher PBF have lower all-cause and cardiovascular mortality, as well as lower N-terminal pro brain natriuretic peptide.10
In conclusion, our data support the notion that not only obese but also overweight patients are at higher risk of ICU admission, and although we did not show an obesity paradox, only severe obesity is associated with an increased risk of mortality in patients invasively ventilated. The results of our study pointed out the need for further studies exploring the role of obesity in patients with SARS-CoV-2 pneumonia using not only BMI but also PBF or ideally a direct measurement of body composition such as the BIVA technique.
Author contribution
GH and MV contributed to the conception or design of the work. MLGL contributed to the acquisition, analysis, or interpretation of data for the work. GH drafted the manuscript. GQV and MN critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work ensuring integrity and accuracy.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team. Vital surveillances: the epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) – China. China CDC Weekly 2020; 2: 113–122 [PMC free article] [PubMed] [Google Scholar]
- 2. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: retrospective cohort study. Lancet 2020; 395: 1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization. WHO Technical Report Series 894. Obesity: preventing and managing the global epidemic Report of a WHO consultation. Geneva: World Health Organization, 2000. [PubMed]
- 4. Parameswaran K, Todd DC, Soth M.. Altered respiratory physiology in obesity. Can Respir J 2006; 13: 203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhi G, Xin W, Ying W, et al. “Obesity paradox” in acute respiratory distress syndrome: asystematic review and meta-analysis. PLoS One 2016; 11: e0163677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Piepoli MF. Obesity in heart failure: is it time to rethink the paradox? Eur J Heart Fail 2017; 19: 1736. [DOI] [PubMed] [Google Scholar]
- 7. Lennon H, Sperrin M, Badrick E, et al. The obesity paradox in cancer: a review. Curr Oncol Rep 2016; 18: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ball L, Serpa Neto A, Pelosi P.. Obesity and survival in critically ill patients with acute respiratory distress syndrome: a paradox within the paradox. Crit Care 2017; 21: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bustamante AF. Adipose–lung cell crosstalk in the obesity-ARDS paradox. J Pulm Respir Med 2013; 3: 144. [Google Scholar]
- 10. Aimo A, Januzzi JL Jr, Vergaro G, et al. Revisiting the obesity paradox in heart failure: per cent body fat as predictor of biomarkers and outcome. Eur J Prev Cardiol 2019; 26: 1751–1759. [DOI] [PubMed] [Google Scholar]