Abstract
Coronavirus disease 2019 (COVID-19), has spread over 210 countries and territories beyond China shortly. On February 29, 2020, the World Health Organization (WHO) denoted it in a high-risk category, and on March 11, 2020, this virus was designated pandemic, after its declaration being a Public Health International Emergency on January 30, 2020. World over high efforts are being made to counter and contain this virus. The COVID-19 outbreak once again proves the potential of the animal-human interface to act as the primary source of emerging zoonotic diseases. Even though the circumstantial evidence suggests the possibility of an initial zoonotic emergence, it is too early to confirm the role of intermediate hosts such as snakes, pangolins, turtles, and other wild animals in the origin of SARS-CoV-2, in addition to bats, the natural hosts of multiple coronaviruses such as SARS-CoV and MERS-CoV. The lessons learned from past episodes of MERS-CoV and SARS-CoV are being exploited to retort this virus. Best efforts are being taken up by worldwide nations to implement effective diagnosis, strict vigilance, heightened surveillance, and monitoring, along with adopting appropriate preventive and control strategies. Identifying the possible zoonotic emergence and the exact mechanism responsible for its initial transmission will help us to design and implement appropriate preventive barriers against the further transmission of SARS-CoV-2. This review discusses in brief about the COVID-19/SARS-CoV-2 with a particular focus on the role of animals, the veterinary and associated zoonotic links along with prevention and control strategies based on One-health approaches.
Keywords: COVID-19, SARS-CoV-2, animals, veterinary, zoonosis, transmission, one health
1. Introduction
In the early days of December 2019, where people planned to welcome New Year 2020, as well as the Chinese New Year, on January 25, 2020, news channels reported suffering of people with sporadic and clustered incidences of “pneumonia of unknown origin” in the city of Wuhan under Hubei province, China (Gao 2020; Lu et al. 2020a). Subsequently, after a month of the first report of infection on December 12, 2019, the causative agent was swiftly identified as a member of Coronaviridae family, and on January 12, 2020, the World Health Organization (WHO) designated this fast-spreading virus as “2019-novel coronavirus (2019-nCoV)”, and Novel Coronaviral Pneumonia and CoV-associated diseases were referred to as “COVID-19” by WHO on February 11, 2020 (Du et al. 2020; Gralinski and Menachery 2020). Later, this emerging virus was designated as “SARS-CoV-2” by the Coronavirus Study Group (CSG) of the International Committee on Taxonomy of Viruses (ICTV) (Gorbalenya et al. 2020). On March 11, 2020 the WHO declared the situation as a pandemic which is threatening mankind to a great extent (Chatterjee et al. 2020; Zheng 2020; Phadke and Saunik 2020; Rundle et al. 2020). As of now, SARS-CoV-2 is considered as the seventh coronavirus that infects humans. The other coronaviruses (CoVs) include HKU1, NL63, OC43, 229E, SARS-CoV, and MERS-CoV. Among which SARS-CoV and MERS-CoV are zoonotic and have resulted in high mortality outbreaks in the last two decades, while the others are usually associated with mild upper-respiratory tract illnesses (Wei et al. 2020), and sometimes leading to complicated disease, when occurring in immunocompromised individuals (Villamil-Gómez et al. 2020).
The culinary habits of Chinese people involve the consumption of wild animal meat. The common motivation that is responsible for the human consumption of wild animal meat in China is due to their believed medicinal value as well as the health-promoting effects associated with the consumption of certain wild game animal meats and their products (Harypursat and Chen 2020). The circumstantial evidence that links the first case of COVID-19 to the Huanan South Seafood Market that sells various exotic live animals and our previous knowledge that coronaviruses are animal-derived made us conclude the possible zoonotic transmission in SARS-CoV-2. Nevertheless, it is too early to jump into conclusions since our knowledge of the primary source of infection is limited (Jalava 2020). Identifying the origin of SARS-CoV-2 will help us to unravel the exact mechanism responsible for its initial transmission. After attaining a remarkable progress in developing field oriented as well as high accuracy lab-based diagnostics, much attention has been paved upon developing effective vaccine and therapeutics for blocking person-person transmission, old age infections and health-care workers infection (Chen et al. 2020). That is critical for developing appropriate preventive and control strategies against the fast-spreading SARS-CoV-2 infection. Looking at the beneficial propositions of hydroxychloroquine a multicentric randomised study is underway to assess its effectiveness as a prophylactic measure in curbing secondary SARS-CoV-2 infections as well as associated clinical symptoms progression reducing overall the spread of the virus (Mitjà and Clotet 2020)
Originating from the central part of China, the SARS-CoV-2 pandemic not only dispersed in 369 other cities of China but also crossed the international boundaries within a short period (December to March 2020). As of May 2, 2020, COVID-2019 has affected persons in more than 210 countries and territories in Asia, Europe, Africa, North America, and Latin America (Rodríguez-Morales et al. 2020a; WHO 2020). Hence, due to very high transmissibility across the borders, it was declared as public health emergency of international concern by the WHO on January 30, 2020, and later as pandemic situation (Du Toit 2020; Habibzadeh and Stoneman 2020; Liu et al. 2020a, 2020b; Wood 2020; WHO 2020).
At the beginning of 21st century, other coronaviruses like SARS-CoV and MERS-CoV, in 2002 and 2012, respectively have also caused severe acute respiratory distress (SARD) in the form of outbreaks but the current SARS-CoV-2 pandemic affected wider population accounting a total number of nearly 4.71 million confirmed cases along with death toll of nearly 0.31 million by May 17, 2020 (WHO 2020). These numbers are comparatively higher than SARS-CoV and MERS-CoV cases but with lower case fatality rate. The occurrence of this pandemic has adversely affected the global economy, especially in developing nations. This outbreak not only dejected the multinational businesses, disrupted the global market trading, tourism, transportation, export-import, but also reduced the income generated from the market (Ayittey et al. 2020).
China is home for several farms that rears several animal species such as deer, snakes, porcupines, foxes, civets, bears, turtles, bamboo rats, mink, and birds. Such farms can be targeted to find the origin of SARS-CoV-2 (Zhai et al. 2020). Before declaring snakes, pangolins or even dogs as the reservoir host of SARS-CoV-2, a set of established principles called as the Koch’s postulates have to be satisfied. Hence, it is unethical to cull these animals without any conclusive evidence of SARS-CoV-2 transmission from animals-to-humans (Brownlie 2020). The recent reports of SARS-CoV-2 in animals such as dogs, cats, and a tiger have resulted in unnecessary fear among the general public as well as pet owners and have negatively impacted the welfare of animals (Parry 2020).
The present compilation highlights, in brief, abut SARS-CoV-2, causing emerging coronavirus disease (COVID-19) in humans with regards to the role of animals, veterinary importance, zoonotic aspects, and salient prevention and control strategies focusing on One-health approaches to restrain and combat this pandemic virus.
2. The virus (SARS-CoV-2)
Coronaviruses are positive-sense RNA viruses. The newly identified SARS-CoV-2 (2019-nCoV) is a member of the order Nidovirales, family Coronaviridae, sub-family Orthocoronavirinae under which four genera, namely, Alphacoronavirus, Betacoronavirus, Gammacoronavirus, and Deltacoronavirus, are categorized. The SARS-CoV-2 belongs to the genus Betacoronavirus and subgenus Sarbecovirus. SARS-CoV and MERS-CoV were also part of the Betacoronavirus genus, but SARS-CoV-2 is different from these two at genetic level. The SARS-CoV-2 has been found 88-89% identical to two bat origin SARS coronaviruses (bat-SL-CoVZC45 and bat-SL-CoVZXC21, also named as ZC45 and ZXC21), while it is 82% identical to human SARS-CoV Tor2 and human SARS-CoV BJ01 2003 at the nucleotide level (Drexler et al. 2014; Hu et al. 2017; Hu et al. 2018; Chan et al. 2020; Malik et al. 2020a). Only 50-51.8% identity was observed between SARS-CoV-2 and MERS-CoV and 79% between SARS-CoV-2 and SARS-CoV; further molecular level phylogenetic analyses reveal that SARS-CoV-2 is more close to bat origin SARS-CoV (Mohd et al. 2016; Ramadan and Shaib 2019; Ren et al. 2020; Malik et al. 2020a). The advanced, in-depth genome analysis identified the presence of 380 amino acid substitutions between the sequences of SARS-CoV-2 (HB01) in comparison to the corresponding consensus sequences of SARS-CoV and SARS-CoV like viruses. This amino acid substitution might have contributed to the functional as well as the pathogenic divergence of this novel virus (Wu et al. 2020a).
3. Host range
Coronaviruses (CoVs) infect man as well as domestic and wild animal species and usually infections remain sub-clinical in most cases (Ji et al. 2020a; Li et al. 2020a; Salata et al. 2020). The clinical form varies from enteritis in cattle, horses and swine, upper respiratory tract disease in cattle, dogs, felines, and poultry, and common cold to highly fatal respiratory infections in humans (Dhama et al. 2020a, 2020b). Among the four genera in the Coronaviridae family, Alphacoronavirus and Betacoronavirus usually infect mammals and have probable bat origin, while Gammacoronavirus and Deltacoronavirus infect birds, fishes, and mammals and are assumed to have swine origin (Woo et al. 2012; Hu et al. 2017; Cui et al. 2019). The genus Betacoronavirus possess potential zoonotic pathogens like SARS-CoV and MERS-CoV which have bats as primary host and palm civet cat and dromedary camels as intermediate hosts, respectively (Wang and Eaton 2007; Ar Gouilh et al. 2018; Ramadan and Shaib 2019). Many CoVs have been recovered from birds such as Wigeon coronavirus HKU20, Bulbul coronavirus HKU11, Munia coronavirus HKU13, White-eye coronavirus HKU16, Night-heron coronavirus HKU19 and Common moorhen coronavirus HKU21. The common pig infecting coronaviruses include Porcine Coronavirus HKU15, Transmissible Gastroenteritis Virus (TGEV), Porcine Epidemic Diarrhea Virus (PEDV), and Porcine Hemagglutinating Encephalomyelitis virus (PHEV) which are being reported from many parts of the world (Ma et al., 2008). A list of other animal species also reported harbouring the CoVs such as cattle, horses, swine, dogs, cats, camels, rabbits, rodents, birds, ferrets, mink, bats, snake (such as Chinese cobra and krait), frogs, marmots, hedgehogs (Erinaceus europaeus), Malayan or Javan or Sunda pangolin (Manis javanica), many other wild animals and their role as carrier/reservoir needs urgent attention (WHO 2003; Dhama et al. 2014a, 2014b, 2020a; Monchatre-Leroy et al. 2017; Ji et al. 2020a; Malik et al., 2020b; Xu 2020).
4. Covid-19/SARS-CoV-2: animals, veterinary, zoonotic links and transmission
4.1. Coronaviruses affecting animals
As coronaviruses have a broad animal host range, several animal species harbour these pathogens, and only a few of them get a severe infection (Cui et al. 2019; Andersen et al. 2020). Coronaviruses like mouse hepatitis virus, rat sialodacryoadenitis coronavirus, guinea pig coronavirus and rabbit coronaviruses are some important CoVs responsible for hepatitis, enteritis, and respiratory infections in lab animals. Among large animals, bovine coronaviruses (BoCoVs) have zoonotic potential as being isolated from asymptomatic children and also found affecting several domestic and wild ruminants, in which calf diarrhea in neonates, bloody diarrhea in adult cattle and respiratory form of shipping fever in all age groups of cattle are universal implications (Zhang et al. 1994; Suzuki et al. 2020). Feline CoVs affect the respiratory tract, central nervous system, abdominal cavity, and gastrointestinal tract to produce enteritis and infectious peritonitis (Tekes and Thiel 2016). Canine enteric coronavirus of Alphacoronavirus and canine respiratory coronavirus of Betacoronavirus genera affect the enteric and respiratory tract, respectively (Erles and Brownlie 2008; Licitra et al. 2014). In the poultry industry, infectious bronchitis virus (IBV), member of the genus Gammacoronavirus cause extensive economic loss by producing respiratory illness, urinary tract infection, and reproductive problems (Dhama et al. 2014a, 2014b). Swine acute diarrhea syndrome coronavirus (SADS-CoV), a member of genus Alphacoronavirus, produces severe enteritis in suckling piglets, causing significant mortality. Upon genomic analysis, SADS-CoV was found 95-96% identical to horseshoe bat origin (Rhinolophus sp.) coronavirus and named as HKU2 coronavirus (Wang and Jin 2020). It suggested the possibility of host jumping by a coronavirus from bats to pigs by crossing the species barrier either by genetic recombination or by making changes at the level of the receptor-binding domain (RBD) (Zhou et al. 2018; Yang et al. 2019).
Among different animal species, another novel CoV named SW1 has been identified by using panviral microarray technology in the liver tissue of the captive beluga whale (Delphinapterus leucas) (Mihindukulasuriya et al. 2008).
4.2. Animals and zoonotic links of SARS-CoV-2
Of note, coronaviruses have crossed the species barrier twice in the past during SARS and MERS outbreaks, and thus SARS-CoV-2 looks to be the outcome of species barrier jumping for the third time. Amongst CoVs, recent zoonotic ones such as SARS-CoV, MERS-CoV, and SARS-CoV-2 gained higher importance due to the severity of disease in humans and their global spread (Rothan and Byrareddy 2020). The emergence of novel CoVs and their wide host range may be due to instability of the replicase enzyme, RNA dependent RNA polymerase, polybasic furin cleavage site, and O-linked glycans, lack of proofreading mechanism, a higher rate of mutations in the RBD of spike gene and genetic recombination (Su et al. 2016; Chen 2020; Patel and Jernigan 2020). Researchers also showed that SARS-CoV and SARS-CoV-2 (2019-nCoV) both use ACE2 as a similar cell entry receptor (Zhou et al. 2020a). Due to the mutation in the RBD region of S gene of CoVs, the host-range get expanded to infect other host species of animals or humans, pathogenicity and transmissibility of virus may further get altered and increased, becoming a matter of global worry (Chen 2020; Patel and Jernigan 2020).
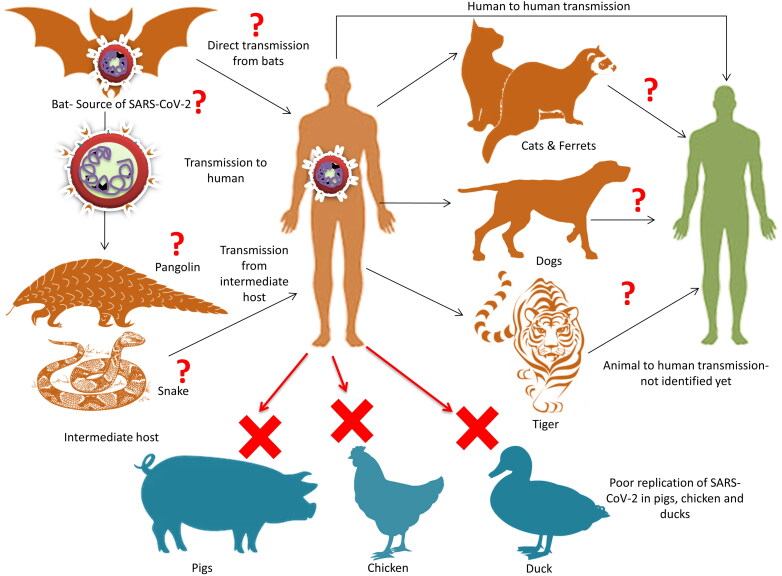
While searching the source of SARS-CoV-2, it was observed that the initially infected individuals had a common exposure spot. It was South China Wet Seafood wholesale market in Wuhan, Hubei Province, China, where restaurants are famous for offering various small and large domestic animals, wild animals, and live animals including poultry, rabbits, bats, snakes, pangolins, turtles, hedgehogs, badgers, and marmots for human consumption (Hu et al. 2015; Hui et al. 2020; Ji et al. 2020b; Liu et al. 2020a, 2020b; Lu et al. 2020b; Wang et al. 2020; Wu et al. 2020b). The initial inferences from Wuhan Seafood Market hypothesised animal source attachments and wild animals for the spillover of SARS-CoV-2. Findings indicated the probability of a zoonotic basis, as CoVs keep on circulating between various animal species, vertebrate, and humans due to a broad host range (Figure 1). It was assumed that SARS-CoV-2 got initially transmitted from animals to humans, followed by maintaining via human-to-human transmission (Hui et al. 2020; Ji et al. 2020a; Nishiura et al. 2020). In the case of MERS-CoV, there is evidence that the viral RNA is not only shed by nasal secretions and feces but also from milk, suggesting the risk of food-borne transmission of MERS-CoV (Reusken et al. 2014). Additionally, a high proportion of camels presenting for slaughter in some studies showed evidence for nasal MERS-CoV shedding (Farag et al. 2015). Further, the possibility of SARS-CoV-2 being the food-borne CoV infection that is transmitted by the respiratory route cannot be rejected (Jalava 2020). Literature documents that a few of the bat origin SARS-CoVs were likely capable of infecting human beings. As seen earlier, bats were found involved in the transmission of SARS-CoV and MERS-CoV, thereby researchers forecasted on the role of bats in the origin and transmission for the current pandemic of SARS-CoV-2 (Fan et al. 2019; Malik et al. 2020a; Wong et al. 2019; Zhou et al. 2020a). For the time being, it is understood that the SARS-CoV-2 is closely related to the bat coronavirus that was isolated from horseshoe bat, the species of bat that is considered to be a maintenance host of previous SARS-related CoVs. Hence, SARS-CoV-2 might have emerged from the sequential recombination occurring between the precursors of SARS-related coronaviruses. Based upon codon usage bias snake SARS-CoV-2 was proposed as the reservoir of SARS-CoV-2 (Ji et al. 2020a). However, later this proposal was contradicted by several researchers. This is the reason for suspecting the presence of an intermediate animal host that is responsible for the zoonotic spill-over to humans (Weiss and Leibowitz 2011; Murdoch and French 2020).
Figure 1.
Zoonotic links of SARS-CoV-2. Bat has been reported as the reservoir source of SARS-CoV-2. The intermediate host is not yet elucidated clearly and presently snake and/or pangolins are reported to the intermediate host. Reports regarding the transmission of SARS-CoV-2 from human to animal have been speculated. Study also shows that SARS-CoV-2 replicate poorly in pig, chicken and duck while ferrets and cats are susceptible.
Similarly, not only from bats, but coronavirus associated with SARS was transmitted from human beings to pigs (Chen et al. 2005). It is pertinent to mention that pigs had been predominant species for the evolution of many new strains of Influenza A virus in the past when present in close association with avian and human species and as bat CoVs are infecting pigs, the possibility of evolution of any new virus involving influenza and corona cannot be excluded including the current scenario of growing SARS-CoV-2 cases, such hypothesis needs explorative studies (Brown 2001; Dhama et al. 2012; Malik et al. 2020a). Provided conditions, at any point in time, pigs which serve as a mixing vessel of influenza viruses (Ma et al. 2009) need to be taken with caution as they remain in proximity with man and domestic-sylvatic cycles involving contact with many wild animals and then the situation may get worsen (Ma et al. 2008). However, for the time being, findings of Shi et al. (2020) have not revealed significant susceptibility of pigs to SARS-CoV-2.
Bats, civets, and camels have been the recent animal carriers of human CoV infections (Cui et al. 2019). Of the latest, bats (Wu et al. 2020b) and pangolins (Zhang et al. 2020a) are considered to be the probable sources of origin of SARS-CoV-2 (Andersen et al. 2020). Still, actual intermediate host and nature of emergence are yet to be explored. Two scenarios of the emergence of SARS-CoV-2 are being projected. First is that natural selection of viruses that may have occurred in an animal host before transmission to humans and the second is that natural selection of viruses has occurred in humans after zoonotic transmission (Andersen et al. 2020). Advanced studies involving cell culture or animal models can help in clarifying these hypotheses (Ge et al. 2013; Andersen et al. 2020).
4.2.1. Bats
Bats are ideal reservoir hosts for CoVs, as viruses are persistently present in bats and being asymptomatic. They travel across the forests in search of food and transmit the virus to a variety of hosts they come in contact with (Fan et al. 2019). In China, bats are not only sold for food purposes in live-animal markets, but they are an integral part of Traditional Chinese Medicine (TCM) and wild bats are used to obtain bat-derived compounds. Although bats have commercial value, they pose a severe risk of acquiring any new zoonotic infection (Riccucci 2012; Wassenaar and Zou 2020). In the current COVID-19 pandemic, laboratory findings confirmed that SARS-CoV-2 is also 96% identical to the bat CoV at the genomic level, and hence bats may be the primary source of this zoonotic spillover (Tang et al. 2006; Rodriguez-Morales et al. 2020b; Zhou et al. 2020a).
4.2.2. Pangolins
Not only from bats, coronavirus has been isolated from Malayan Pangolins also, and RBD in S protein of SARS-CoV-2 was nearly the same as that of Pangolin-CoV, and thus pangolins might be the intermediate host of SARS-CoV-2 (Xiao et al. 2020). This is also supported by the findings of Zhang et al. (2020a). Interestingly, the coronavirus isolated from pangolins (SRR10168377 and SRR10168378) did not have the RRAR motif. The SARS-CoV-2 virus isolated from the infected individuals showed higher similarity to the Beta CoV/bat/Yunnan/RaTG13/2013 virus compared to the ones that were isolated from the pangolins (Li et al. 2020b). These findings suggested that the pangolins had less probability of acting as the intermediate host of SARS-CoV-2. Further studies are required to identify the intermediate host and to confirm their role in the origin of SARS-CoV-2 in humans.
4.2.3. Canines and felines
Till now, SARS-CoV-2 infection has been noticed in two dogs; both are reported from Hong Kong (Almendros 2020a). The first case was reported in a 17-year-old Pomeranian dog that gave positive RT-PCR results in both oral and nasal samples (Almendros 2020a; American Veterinary Medical Association 2020). Even though the initial serological test gave a negative result, the blood samples taken in the later stages gave weak positive results. This might be due to the fact that formation of antibody can take 14 days or more (Almendros and Gascoigne 2020b). A report of seroconversion in dogs indicates that the animal has produced antibodies against SARS-CoV-2. This is suggestive of a weak infection in the dog that resulted in an immune response. Hence, the findings are suggestive of a true infection in dogs caused by human-to-animal transmission (Almendros and Gascoigne 2020b). Similarly, another case of SARS-CoV-2 infection was reported in a German Shepherd Dog in Hong Kong. It is interesting to note that both the cases of canine SARS-CoV-2 infections were reported in dogs that were living in close contact with SARS-CoV-2 positive owners (American Veterinary Medical Association 2020). Currently, there is no substantial evidence that dogs get SARS-CoV-2 infection, or can transmit this virus to human beings (Almendros 2020a).
Two cats, one from Belgium and another from Hong Kong, were also tested positive for SARS-CoV-2 (American Veterinary Medical Association 2020). The scientists from Harbin Veterinary Research Institute have reported that cats can get infection with SARS-CoV-2 under experimental conditions and can transmit to other susceptible cats that are housed together (Mallapaty 2020; Shi et al. 2020). The findings are based on experimental inoculation and may not be accurate in natural conditions. None of the infected cats showed any signs of illness, indicating the low potential for transmitting the infection (Mallapaty 2020). A serological study was conducted among the cats of Wuhan that observed the presence of SARS-CoV-2 neutralizing antibodies. This indicates that cats can get SARS-CoV-2 infection under natural conditions resulting in an antibody response (Zhang et al. 2020b). However, among the cats that tested positive, a higher titre of neutralizing antibodies was seen in the cats that were living in close contact with SARS-CoV-2 positive owners (Zhang et al. 2020b). Recently, a Malayan tiger maintained in the Bronx Zoo of New York City, NY, USA was also tested positive for SARS-CoV-2. The “Big cat” is suspected to be infected by SARS-CoV-2 positive asymptomatic zookeeper. These carnivores were tested for SARS-CoV-2 when they started showing signs of mild respiratory illness (United States Department of Agriculture 2020).
4.3. Human-animal interactions as a risk factor
Some researchers opined that traditional cooking practices of China are also responsible to some extent for the occurrence of novel CoV infection in humans because as per the Chinese food customs live-slaughtered animals are considered more nutritious, but at the same time, people get exposure to possibly all/any types of pathogens (SARS-CoV, Nipah virus, Hepatitis A virus, Hepatitis E virus, Norovirus, Rotavirus, Highly Pathogenic Avian Influenza virus) present in the offered food items (FAO/WHO 2008). Repeated human-animal interactions either in the market or in the animal industry without using proper environmental biosecurity were considered as the significant risk factors for the emergence of zoonotic diseases, particularly in the rural communities of southern China (Daszak 2020). After these reports, China has put temporarily ban on the sale of wildlife and trading of bats following CoV infection. Furthermore, Wuhan animal food market is also kept closed so that further zoonotic transmission of SARS-CoV-2 and evolution of any new viral variant can be prevented. It is also recommended to avoid any contact with farm or wild animals without the use of personal protective equipment (Benvenuto et al. 2020). Now there is need to draft surveillance strategies and preventive guidelines to have in-depth analysis of bat origin Betacoronavirus, especially in the Rhinolophus bat family as in the past SARS, MERS, and now SARS-CoV-2 epidemic have created panic, and from epidemic, it has turned to pandemic (Daszak et al. 2020).
In a nutshell, bats appears as the natural reservoir or source of origin for SARS-CoV-2 (Li et al. 2020) that causes zoonotic infection in humans through an intermediate host yet to be deciphered with recent investigations on pangolins, ferrets and possibly snakes. However, the future explorations might reveal the actual intermediate host of SARS-CoV-2 responsible for zoonotic transmission (Almendros 2020a; Dhama et al. 2020d; Shi et al. 2020; Zhang et al. 2020a, 2020b).
5. Animal models
Though animal model studies are currently lacking for SARS-CoV-2, a recent study explored the utility of non-human primates, rhesus macaque, as a model for carrying out SARS-CoV-2 studies. Earlier, these non-human primates were used in evaluating the vaccines and antivirals against the MERS-CoV (de Wit et al. 2020). While working on SARS-CoV-2, rhesus macaques showed the establishment of SARS-CoV-2 infection with detection of high virus amount in oral-naso and rectal swabs. The apparent lesions of disease in lung radiographs and clinical signs lasting for up to 16 days proved the effectiveness of the model in studying the pathogenesis of this disease and aiding further in developing and testing vaccines and antivirals (Munster et al. 2020).
Isolation SARS-CoV-2 from dogs is also reported (OIE. 2020). Most recently, Shi et al. (2020) have demonstrated the susceptibility of ferrets, cats, dogs, and different domestic animal species to SARS-CoV-2 by experimental inoculation and reported that SARS-CoV-2 replicates poorly in dogs, pigs, chickens, and ducks, but efficiently in ferrets and cats. Cats can spread infection via droplets (Shi et al. 2020). However, accurate exploration requires specific animal models, especially animals with ACE2 receptors similar to those of humans (Andersen et al. 2020). Establishing suitable animal models will not only help in understanding the disease process but will also help in developing prophylactics and therapeutics (TBRI 2020; Dhama et al. 2020c). Non-human primates are considered to be the appropriate models of human diseases, whereas for exploring etio-pathogenesis of the disease and immune response, other animal models are preferred (TBRI 2020). Non-human primates, mice, and hamsters (Gretebeck and Subbarao 2015) have been used as animal models for SARS and MERS, and some may have the possibility in SARS-CoV-2. Golden Syrian hamsters have been investigated for vaccine protection studies against SARS-CoV strains (Roberts et al. 2008), and suggested to be potential animal model for revealing CoV pathology and pathogenesis along with vaccine efficacy to be tested. Transgenic animals (e.g., mice) have better relevance of simulating SARS-CoV-2 since there are structural differences in ACE 2 receptors in various animal species to which receptor binding domain of spike protein of SARS-CoV-2 binds (Liu et al. 2020b; Wang 2020). On the modeling of ACE2 receptors between various animal species of pigs, ferrets, cats, orangutans, monkeys, bat species, and humans have similar levels of affinity for SARS-CoV-2 based on the structural similarity of their ACE2 receptors (Jarvis 2020). Hence these may have a possible role to be used as animal models with further investigative studies. Small animal models are generally preferred, like mice and rabbits being cheap, easy to manipulate, and ease of availability (Dhama et al. 2020c). Initially, mice appeared to be challenging owing to the difference in receptor ACE2 usage pattern, but transgenic mice are now believed to be relevant models for SARS-CoV-2 (Liu et al. 2020a, 2020b Wang 2020,; Zhou et al. 2020b). Ace2 Knockout Mouse, Tmprss2 Knockout Mouse, Stat1 Knockout Mouse, inbred mice, and Transgenic HLA Mice are being evaluated as animal models for SARS-CoV-2/COVID-19 (Hoffmann et al. 2020; Taconic Biosciences 2020; Wang 2020).
6. Prevention and control
The past decade and recent episodes of Zika, Ebola, Nipah, and Bird flu viruses (Munjal et al. 2017; Dhama et al. 2012, 2018; Singh et al. 2019), and lessons learned from previous threats of coronaviruses (SARS- and MERS-CoVs) along with advances in science have paved pace to counter emerging pathogens including the SARS-CoV-2. For this purpose, high efforts are being made to contain and control the spread of this emerging virus haunting the lives of humans and posing even now a pandemic situation. Efforts for rapid diagnosis, strict vigilance, appropriate isolation, and quarantine procedures to halt its further spread, enhanced surveillance and monitoring, strengthening of medical facilities and intensive care units, networking programs, rapid communication and providing updates, knowledge awareness of its public health risks to the general population, high efforts to develop effective vaccines and therapeutics/drugs are being explored optimally. International collaborative efforts and readiness to tackle further heightened emergency to a level of pandemic potential along with following suitable One health approach to combat this emerging virus haunting the lives of billions of human population are being followed effectively (Bonilla-Aldana et al. 2020; Dhama et al. 2013a, 2013b; 2020a, 2020c; Malik et al. 2020a, 2020b; Rodriguez-Morales et al. 2020c). Vaccines appear to be the long-lasting solution for the COVID-19 pandemic. However, currently, there are no vaccines available against it (Shang et al. 2020; Chen et al. 2020). Clues are being taken from the viral structures, pathogenesis, and related coronaviruses (Ahmed et al. 2020; Shang et al. 2020).
Various vaccines are being evaluated by different institutes and companies (Shang et al. 2020), with a few under trials. Moderna, Cambridge, Ma, USA, a biopharma company, started with the mRNA-1273 vaccine in collaboration with CanSino, Hong Kong Special Administrative Region, China (Flanagan 2020). The University of Alabama at Birmingham (UAB), Birmingham, Al, USA, in coordination with Altimmune Inc., Gaithersburg, Md, USA, is developing an intranasal vaccine against COVID-19 and named it as AdCOVID on the pattern of pandemic influenza vaccine and inhalation anthrax vaccine (Hansen et al. 2020). The Clover Biopharmaceuticals company, Chengdu, China, has come out with a SARS-CoV-2 S-protein based subunit vaccine (Clover Biopharmaceuticals 2020).
As of March 20, 2020, WHO has tabulated approximately 44 vaccine candidates targeting SARS-CoV-2, among which few are under clinical evaluation and some under development by various companies and institutions. They included live attenuated, formaldehyde inactivated, protein subunit, DNA, m-RNA, VLP, replicating, and non-replicating vector-based SARS-CoV-2 vaccines. Adenovirus type 5 vector vaccine by CanSino biological Inc., Hong Kong Special Administrative Region, China, and Beijing Institute of Biotechnology, Beijing, China, and LNP-encapsulated mRNA vaccine developed by Moderna/NIAID, Bethesda, Md, USA, is under phase-1 clinical evaluation. Few in pre-clinical stage of clinical evaluation against COVID-19 include DNA plasmid vaccine by Zydus Cadila, Ahmedabad, India, DNA plasmid vaccine through electroporation device by Inovio pharmaceuticals, Plymouth Meeting, Pa, USA, DNA vaccine by Takis/Applied DNA Sciences/Evvivax, Rome, Italy, formaldehyde inactivated alum vaccine by Sinovac, Beijing, China, live attenuated virus vaccine by Codagenix, Farmingdale, New York, USA/Serum Institute of India, Pune, India, MVA encoded VLP by GeoVax/BravoVax, Smyrna, Ga, USA, Ad 26 by Janssen pharmaceutical companies, Beerse, Belgium, ChAdOx1 by university of Oxford, Oxford, UK, adenovirus-based NasoVAX expressing SARS-CoV-2 spike protein by Altimmune, Gaithersburg, Md, USA, Ad5 S (named as GREVAX™) by Greffex, Aurora, Co, USA, oral vaccine by Vaxart, South San Francisco, Ca, USA, Drosophila S2 insect cell expression system using VLPs as protein subunit vaccine by ExpreS2ion, Horsholm, Denmark, S protein based by WRAIR/USAMRIID, Fort Detrick, Md, USA, another S protein by AJ Vaccines, København, Denmark, S-trimer by Clover Biopharmaceuticals Inc., Chengdu, China/GSK, Brentford, UK, peptide based vaccine by Vaxil Bio, Toronto, Ontario, Canada, S protein through baculovirus production system by Sanofi Pasteur company, Swiftwater, Pa, USA, full length S trimers nanoparticle with Matrix M by Novavax, Rockville, Md, USA, gp-96 backbone based vaccine by Heat Biologics, Morrisville, Nc, USA, or University of Miami, Miami, Fl, USA, S1 or RBD protein based by Baylor College of Medicine, Houston, Tx, USA, adjuvanted microsphere peptide vaccine candidate by University of Saskatchewan, Saskatoon, Saskatchewan, Canada, LNP encapsulated mRNA encoding RBD by Fudan University/Shanghai JiaoTong University/RNACure Biopharma, Shanghai, China, sa-RNA (small activating ds-RNA) based COVID-19 vaccine by Imperial College London, London, UK, among others. (https://www.who.int/blueprint/priority-diseases/key-action/novel-coronavirus-landscape-ncov.pdf?ua=1). Though till April 1st, 2020 US Food and Drug Administration (FAO) had not announced any confirmed commercial therapeutic or prophylactic vaccine against SARS-CoV-2, nevertheless they have enlisted few potential vaccine candidates which are currently under either preclinical or clinical trials such as mRNA-1273 by Moderna Inc., Bethesda, Md, USA, Inovio’s DNA vaccine INO-4800 against COVID-19 by Inovio Pharmaceuticals, Plymouth Meeting, Pa, USA, along with Ology Bioservices, Alachua, Fl, USA, BNT162 a mRNA vaccine by BioNTech, Mainz, Germany, plant-based COVID-19 vaccine by Medicago, Quebec, Quebec, Canada, oral recombinant COVID-19 vaccine by Vaxart, South San Francisco, Ca, USA, Ii-Key peptide COVID-19 vaccine by Generex Biotechnology, Toronto, Ontario, Canada, among others (Precision Vaccinations 2020). (https://www.precisionvaccinations.com/vaccines/coronavirus-vaccines).
Till the vaccines are developed, alternate disease prevention and control strategies need to be focused on. There is a need for strengthening infrastructure and capacity building with a trained workforce, hospital, health workers to identify the SARS affected patient with the isolation of patients after being doubted for COVID-2019. To apply any prevention, measure the first and foremost step is to diagnose the case with accuracy and speed. While confirming any deadly case, the guidelines of Centers for Disease Control and Prevention (CDC) must be adopted. As the suspected case is a good source of nosocomial spread, the health workers must follow all the precautionary practices while handling the COVID-2019 case. Notably, a facility with negative air pressure is recommended for keeping confirmed patients of SARS-CoV-2.
The applications of telemedicine having tele-visits and supervision, monitoring, interpretation, and consultation (Serper and Volk 2018) have proven effective in mitigating chronic diseases. The tele-model has been applied on present-day COVID-19 pandemic, especially in the remote areas having limited medical backups saving both manpower as well as resources (Au 2020).
Although respiratory tract infections and spread of the virus from naso-oral secretions is well discussed, the first isolation of SARS-CoV-2 in China from a COVID-19 positive patient highlighted the significance of the digestive tract in conjunction to the respiratory tract. Since then, while planning the control strategies, this alternative route of virus spread must be kept in mind, including a focus on clinical sufferers, and asymptomatic patients or individuals having no or mild signs (Gu et al. 2020).
Further spread of SARS-CoV-2 can be controlled by immediate isolation of confirmed cases along with contact tracing. According to the analysis made by using transmission models, the COVID-19 outbreak could be controlled within three months by following active contact tracing in combination with the isolation of infected individuals (Hellewell et al. 2020). It is not the first time that live-animal markets in China have been identified as the epicenter of emerging novel zoonotic diseases. For preventing the likelihood of another zoonotic outbreak, closure of all the live-animal markets is a necessity (Peeri et al. 2020). However, the deep connection existing between these live markets and the Chinese culture makes the permanent ban practically impossible. Due to the possible role played by live-markets in the origin of SARS-CoV-2, a temporary ban was imposed on the wildlife trade in China (Harypursat and Chen 2020; Mallapaty 2020), which was again cancelled merely after 4 months of the outbreak as COVID-19 seemed under control in China. Purchase of live-dead animals like dogs, cats, bats, birds, ostriches, rabbits, hamsters, scorpions, badgers, pangolins (with peculiar scaly anteaters), minks, palm civet, snapping turtles, ducks, fishes, Siamese crocodiles (Knowles 2020) (https://www.dailymail.co.uk/news/article-8163761/Chinese-markets-selling-bats.html) again started in more than hundreds of animal markets without imposing strict hygienic preventive measures, and this may again be a predisposing factor for recurrence of health ailment due to COVID-19 in China (Anonymous 2020) (https://metrosaga.com/wuhan-virus-chinese-animal-markets-reopened-with-almost-no-precautions/). A bold decision on the future of wild animal trade in China is a necessity to prevent possible future outbreaks due to zoonotic spillover and hence International agencies like WHO, FAO and OIE must frame strict guidelines with regulations and conditional penalties on purchase of food animals in markets for safer future (Blakeman 2020)(https://thehill.com/opinion/international/490528-china-must-close-down-wet-markets-now). A total ban on the wild animal trade will affect the livelihood of a substantial proportion of the population. Hence such a move will only shift the trade of wild animals to the black market (Mallapaty 2020).
The significance of rapid sharing of the technical facts during the COVID-19 outbreak situations in minimizing the panic perpetuation in public was assessed recently by Song and Karako (2020). It was pointed out that scientific data sharing further enriches the epidemiologists, healthcare workers, and forecasting models to comprehend the usefulness of available interferences from new real-time information (Song and Karako 2020). Furthemore, Habibi et al. (2020) emphasized the significance of following International Health Regulations (IHR) measures to defeat the spread of SARS-CoV-2 across the globe (Zhang et al., 2020).
7. Conclusion and future prospects
The live-animal markets, just like the Huanan South China Seafood Market, will continue to act as an ideal point that promotes inter-species contact between the wild and domestic animal species. Hence, the possibility of inter-species transmission of CoV infections at such hot spots is a point of concern to human beings due to the adaptive genetic recombination that occurs in these viruses. The permanent ban on the wild animal trade should not be implemented as it will only shift the trade to the black market. Rather than going for the complete ban, it is better to regulate the trade of wild animal species all around the country. The emergence of newer zoonotic infections like SARS-CoV-2 is inevitable in the future. Hence, local and international regulatory authorities need to develop and implement robust disease control mechanisms that effectively decrease the possibility of human exposure to wild animals. The SARS-CoV-2 outbreak is just another critical example that proves the existence of a close but straightforward interaction between humans, animals, and the environmental health that can potentially result in the emergence of a deadly pandemic. The past decades have shown us the destructive potential of several zoonotic coronavirus infections like SARS, MERS, and now SARS-CoV-2 that calls for the implementation of One Health as a framework to protect humankind from emerging pathogens soon.
Acknowledgments
All the authors acknowledge and thank their respective Institutes and Universities.
Disclosure statement
All authors declare that there exist no commercial or financial relationships that could, in any way, lead to a potential conflict of interest.
References
- Ahmed SF, Quadeer AA, McKay MR.. 2020. Preliminary identification of potential vaccine targets for the COVID-19 Coronavirus (SARS-CoV-2) based on SARS-CoV immunological studies. Viruses. 12(3):254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almendros A. 2020. a. Can companion animals become infected with Covid-19? Vet Rec. 186(12):388–389. [DOI] [PubMed] [Google Scholar]
- Almendros A, Gascoigne E.. 2020. b. Can companion animals become infected with Covid-19? Vet Rec. 186(13):419–420. [DOI] [PubMed] [Google Scholar]
- American Veterinary Medical Association . 2020. SARS-CoV-2 in animals, including pets. [Accessed 2020 April 11]. https://www.avma.org/resources-tools/animal-health-and-welfare/covid-19/sars-cov-2-animals-including-pets.
- Andersen KG, Rambaut A, Lipkin WI, Holmes EC, Garry RF.. 2020. The proximal origin of SARS-CoV-2. Nat Med. 26(4):450–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ar Gouilh M, Puechmaille SJ, Diancourt L, Vandenbogaert M, Serra-Cobo J, Lopez Roig M, Brown P, Moutou F, Caro V, Vabret A, EPICOREM consortium, et al. 2018. SARS-CoV related Betacoronavirus and diverse Alphacoronavirus members found in western old-world. Virology. 517:88–97.,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au S. 2020. Revisiting the role of telemedicine under the 2019 novel coronavirus outbreak. EJGG. 2(1):26–27. [Google Scholar]
- Ayittey FK, Ayittey MK, Chiwero NB, Kamasah JS, Dzuvor C.. 2020. Economic impacts of wuhan 2019-nCoV on China and the world. J Med Virol. 92(5):473–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benvenuto D, Giovanetti M, Ciccozzi A, Spoto S, Angeletti S, Ciccozzi M.. 2020. The 2019-new coronavirus epidemic: evidence for virus evolution. J Med Virol. 92(4):455–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilla-Aldana DK, Dhama K, Rodriguez-Morales AJ.. 2020. Revisiting the one health approach in the context of COVID-19: a look into the ecology of this emerging disease. Adv Anim Vet Sci. 8(3):234–237. [Google Scholar]
- Brown IH. 2001. The pig as an intermediate host for influenza a viruses between birds and humans. Int Congr Ser. 1219:173–178. [Google Scholar]
- Brownlie J. 2020. Conclusive proof needed for animal virus reservoirs. Vet Rec. 186(11):354–354. [DOI] [PubMed] [Google Scholar]
- Chan JF, Kok KH, Zhu Z, Chu H, To KK, Yuan S, Yuen KY.. 2020. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect. 9(1):221–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Yan M, Yang L, Ding B, He B, Wang Y, Liu X, Liu C, Zhu H, You B, et al. 2005. SARS-associated coronavirus transmitted from human to pig. Emerg Infect Dis. 11(3):446–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee P, Nagi N, Agarwal A, Das B, Banerjee S, Sarkar S, Gupta N, Gangakhedkar RR.. 2020. The 2019 novel coronavirus disease (COVID-19) pandemic: A review of the current evidence. Indian J Med Res. 0(0):0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. 2020. Pathogenicity and transmissibility of 2019-nCoV-A quick overview and comparison with other emerging viruses. Microbes Infect. 22(2):69–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WH, Strych U, Hotez PJ, Bottazzi ME.. 2020. The SARS-CoV-2 vaccine pipeline: an overview. Curr Trop Med Rep. :1–4. doi: 10.1007/s40475-020-00201-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2020. China bans sale of wildlife following coronavirus. Vet Rec. 186(5):144–145. [DOI] [PubMed] [Google Scholar]
- Clover Biopharmaceuticals. 2020. Clover initiates development of recombinant subunit-trimer vaccine for Wuhan coronavirus (2019-nCoV). Available at: https://pipelinereview.com/index.php/2020012873644/Vaccines/Clover-Initiates-Development-of-Recombinant-Subunit-Trimer-Vaccine-for-Wuhan-Coronavirus-2019-nCoV.html
- Cui J, Li F, Shi ZL.. 2019. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 17(3):181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daszak P. 2020. A qualitative study of zoonotic risk factors among rural communities in southern China. Int Health. 12(2):77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daszak P, Olival K, Li H.. 2020. A strategy to prevent future epidemics similar to the 2019-nCoV outbreak. Biosaf Health. 2(1):6–8. 10.1016/j.bsheal.2020.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit E, Feldmann F, Cronin J, Jordan R, Okumura A, Thomas T, Scott D, Cihlar T, Feldmann H.. 2020. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc Natl Acad Sci Usa. 117(12):6771–6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhama K, Verma AK, Rajagunalan S, Deb R, Karthik K, Kapoor S, Mahima Tiwari R, Panwar PK, Chakraborty S.. 2012. Swine flu is back again: A review. Pak J Biol Sci. 15(21):1001–1009. [DOI] [PubMed] [Google Scholar]
- Dhama K, Chakraborty S, Kapoor S, Tiwari R, Kumar A, Deb R, Rajagunalan S, Singh R, Vora K, Natesan S.. 2013. a. One world, one health - veterinary perspectives. Adv Anim Vet Sci. 1(1):5–13. [Google Scholar]
- Dhama K, Verma AK, Tiwari R, Chakraborty S, Vora K, Kapoor S, Deb R, Karthik K, Singh R, Munir M, et al. 2013. b. A perspective on applications of geographical information system (GIS); an advanced tracking tool for disease surveillance and monitoring in veterinary epidemiology. Adv Anim Vet Sci. 1(1):14–24. [Google Scholar]
- Dhama K, Pawaiya RVS, Chakrabort S, Tiwari R, Saminathan M, Verma AK.. 2014. a. Coronavirus infection in equines: a review. Asian J Anim Vet Adv. 9(3):164–176. [Google Scholar]
- Dhama K, Singh SD, Barathidasan R, Desingu PA, Chakraborty S, Tiwari R, Kumar MA.. 2014. b. Emergence of avian infectious bronchitis virus and its variants need better diagnosis, prevention and control strategies: a global perspective. Pak J Biol Sci. 17(6):751–767. [DOI] [PubMed] [Google Scholar]
- Dhama K, Karthik K, Khandia R, Chakraborty S, Munjal A, Latheef SK, Kumar D, Ramakrishnan MA, Malik YS, Singh R, et al. 2018. Advances in designing and developing vaccines, drugs, and therapies to counter Ebola virus. Front Immunol. 9:1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhama K, Sharun K, Tiwari R, Sircar S, Bhat S, Malik YS, Singh KP, Chaicumpa W, Bonilla-Aldana DK, Rodriguez-Morales AJ.. 2020. a. Coronavirus disease 2019 – COVID-19. Preprints. doi: 10.20944/preprints202003.0001.v2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhama K, Patel SK, Pathak M, Yatoo MI, Tiwari R, Malik YS, Singh R, Sah R, Rabaan AA, Bonilla-Aldana DK, et al. 2020. b. An update on SARS-COV-2/COVID-19 with particular reference on its clinical pathology, pathogenesis, immunopathology and mitigation strategies – a review. Preprints. doi: 10.20944/preprints202003.0348.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhama K, Sharun K, Tiwari R, Dadar M, Malik YS, Singh KP, Chaicumpa W.. 2020. c. COVID-19, an emerging coronavirus infection: advances and prospects in designing and developing vaccines, immunotherapeutics, and therapeutics. Hum Vaccin Immunother. 18:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhama K, Patel SK, Sharun K, Pathak M, Tiwari R, Yatoo MI.. 2020. d. SARS-CoV-2: Jumping the species barrier, lessons from SARS and MERS, its zoonotic spillover, transmission to humans, preventive and control measures and recent developments to counter this pandemic virus. Preprints. doi: 10.20944/preprints202004.0011.v1. [DOI] [Google Scholar]
- Drexler JF, Corman VM, Drosten C.. 2014. Ecology, evolution and classification of bat coronaviruses in the aftermath of SARS. Antiviral Res. 101:45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du B, Qiu HB, Zhan X, Wang YS, Kang HYJ, Li XY, Wang F, Sun B, Tong ZH.. 2020. Pharmacotherapeutics for the new coronavirus pneumonia. Zhonghua Jie He He Hu Xi Za Zhi. 43(0):E012. [DOI] [PubMed] [Google Scholar]
- Du Toit A. 2020. Outbreak of a novel coronavirus. Nat Rev Microbiol. 18(3):123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erles K, Brownlie J.. 2008. Canine respiratory coronavirus: an emerging pathogen in the canine infectious respiratory disease complex. Vet Clin North Am Small Anim Pract. 38(4):815–825, viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Zhao K, Shi ZL, Zhou P.. 2019. Bat coronaviruses in China. Viruses. 11(3):210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Agriculture Organization of the United Nations/World Health Organization (FAO/WHO) . 2008. Microbiological hazards in fresh leafy vegetables and herbs: meeting report. Microbiological Risk Assessment Series No. 14. Rome. 151.
- Farag EABA, Reusken CBEM, Haagmans BL, Mohran KA, Stalin Raj V, Pas SD, Voermans J, Smits SL, Godeke G-J, Al-Hajri MM, et al. 2015. High proportion of MERS-CoV shedding dromedaries at slaughterhouse with a potential epidemiological link to human cases, Qatar 2014. Infect Ecol Epidemiol. 5:28305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan C. 2020. First results from moderna covid-19 vaccine may take two more months. [Accessed 2020 April 2]. https://www.bloomberg.com/news/articles/2020-03-26/first-look-at-moderna-covid-19-vaccine-may-take-two-more-months.
- Gao ZC. 2020. [Efficient management of novel coronavirus pneumonia by efficient prevention and control in scientific manner]]. Zhonghua Jie He He Hu Xi Za Zhi. 43(0):E001. [DOI] [PubMed] [Google Scholar]
- Ge XY, Li JL, Yang XL, Chmura AA, Zhu G, Epstein JH, Mazet JK, Hu B, Zhang W, Peng C, et al. 2013. Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature. 503(7477):535–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbalenya AE, Baker SC, Baric RS, de Groot RJ, Drosten C, Gulyaeva AA, Haagmans BL, Lauber C, Leontovich AM, Neuman BW, et al. 2020. The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming It SARS-CoV-2.. Nat Microbiol. 5(4):536–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gralinski LE, Menachery VD.. 2020. Return of the coronavirus: 2019-nCoV. Viruses. 12(2):135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gretebeck LM, Subbarao K.. 2015. Animal models for SARS and MERS coronaviruses. Curr Opin Virol. 13:123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J, Han B, Wang J.. 2020. COVID-19: Gastrointestinal manifestations and potential fecal-oral transmission. Gastroenterology. 158(6):1518–1519. [published online ahead of print, 2020 Mar 3]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habibi R, Burci GL, de Campos TC, Chirwa D, Cinà M, Dagron S, Eccleston-Turner M, Forman L, Gostin LO, Meier BM, et al. 2020. Do not violate the international health regulations during the COVID-19 outbreak. Lancet. 395(10225):664–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habibzadeh P, Stoneman EK.. 2020. The novel coronavirus: a bird's eye view. Int J Occup Environ Med. 11(2):65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J, Brown W, Robinson A.. 2020. UAB will test a COVID-19 vaccine candidate created by Altimmune Inc. [Accessed 2020 April 1]. https://www.uab.edu/news/research/item/11203-uab-will-test-a-covid-19-vaccine-candidate-created-by-altimmune-inc.
- Harypursat V, Chen YK.. 2020. Six weeks into the 2019 coronavirus disease (COVID-19) outbreak- it is time to consider strategies to impede the emergence of new zoonotic infections. Chin Med J (Engl). 133(9):1118–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellewell J, Abbott S, Gimma A, Bosse NI, Jarvis CI, Russell TW, Munday JD, Kucharski AJ, Edmunds WJ, Funk S, et al. 2020. Feasibility of controlling COVID-19 outbreaks by isolation of cases and contacts. Lancet Glob Health. 8(4):e488–e496., [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S.. 2020. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 181(2):271–280.e8. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. 2020.DRAFT landscape of COVID-19 candidate vaccines – 20 April 2020. https://www.who.int/blueprint/priority-diseases/key-action/novel-coronavirus-landscape-ncov.pdf?ua=1.
- Precision Vaccinations. 2020.Coronavirus Vaccines. https://www.precisionvaccinations.com/vaccines/coronavirus-vaccines.
- Knowles G. 2020. Will they ever learn? Chinese markets are still selling bats and slaughtering rabbits on blood-soaked floors as Beijing celebrates 'victory' over the coronavirus. Mail Online. https://www.dailymail.co.uk/news/article-8163761/Chinese-markets-selling-bats.html.
- Anonymous. 2020. Wuhan Virus: Chinese Animal Markets Reopened With Almost No Precautions. Metrosaga. https://metrosaga.com/wuhan-virus-chinese-animal-markets-reopened-with-almost-no-precautions/.
- Blakeman BA. 2020. China must close down 'wet markets' now. The Hill. https://thehill.com/opinion/international/490528-china-must-close-down-wet-markets-now.
- Hu B, Ge X, Wang LF, Shi Z.. 2015. Bat origin of human coronaviruses. Virol J. 12:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Zeng LP, Yang XL, Ge XY, Zhang W, Li B, Xie JZ, Shen XR, Zhang YZ, Wang N, Luo DS, et al. 2017. Discovery of a rich gene pool of bat SARS-related coronaviruses provides new insights into the origin of SARS coronavirus. PLoS Pathog. 13(11):e1006698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D, Zhu C, Ai L, He T, Wang Y, Ye F, Yang L, Ding C, Zhu X, Lv R, et al. 2018. Genomic characterization and infectivity of a novel SARS-like coronavirus in Chinese bats. Emerg Microbes Infect. 7(1):154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui DS, I Azhar E, Madani TA, Ntoumi F, Kock R, Dar O, Ippolito G, Mchugh TD, Memish ZA, Drosten C, et al. 2020. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health - The latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 91:264–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalava K. 2020. First respiratory transmitted food borne outbreak? Int J Hyg Environ Health. 226:113490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis C. 2020. Mar 16, 2020 which species transmit Covid-19 to humans? we’re still not sure. [Accessed 2020 April 1). https://www.the-scientist.com/news-opinion/which-species-transmit-covid-19-to-humans-were-still-not-sure-67272.
- Ji W, Wang W, Zhao X, Zai J, Li X.. 2020. a. Cross-species transmission of the newly identified coronavirus 2019-nCoV. J Med Virol. 92(4):433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji W, Wang W, Zhao X, Zai J, Li X.. 2020. b. Homologous recombination within the spike glycoprotein of the newly identified coronavirus may boost cross-species transmission from snake to human. J Med Virol. 92 (4):433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Song Y, Wong G, Cui J.. 2020. a. Bat origin of a new human coronavirus: there and back again. Sci China Life Sci. 63(3):461–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zai J, Zhao Q, Nie Q, Li Y, Foley BT, Chaillon A.. 2020. b. Evolutionary history, potential intermediate animal host, and cross-species analyses of SARS-CoV-2. J Med Virol. 92(6):602–611. 10.1002/jmv.25731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licitra BN, Duhamel GE, Whittaker GR.. 2014. Canine enteric coronaviruses: emerging viral pathogens with distinct recombinant spike proteins. Viruses. 6(8):3363–3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zheng X, Tong Q, Li W, Wang B, Sutter K, Trilling M, Lu M, Dittmer U, Yang D.. 2020. a. Overlapping and discrete aspects of the pathology and pathogenesis of the emerging human pathogenic coronaviruses SARS-CoV, MERS-CoV, and 2019-nCoV. J Med Virol. 92(5):491–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Xiao X, Wei X, Li J, Yang J, Tan H, Zhu J, Zhang Q, Wu J, Liu L.. 2020. b. Composition and divergence of coronavirus spike proteins and host ACE2 receptors predict potential intermediate hosts of SARS-CoV-2. J Med Virol. 92(6):595–601. 10.1002/jmv.25726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Stratton CW, Tang YW.. 2020. a. Outbreak of pneumonia of unknown etiology in Wuhan China: the mystery and the miracle. J Med Virol. 92(4):401–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu N, et al. 2020. b. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 395(10224):565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W, Lager KM, Vincent AL, Janke BH, Gramer MR, Richt JA. The role of swine in the generation of novel influenza viruses. Zoon Pub Health. 2009;56(6-7):326–337. [DOI] [PubMed] [Google Scholar]
- Ma W, Kahn R E, Richt J A.. 2008. The pig as a mixing vessel for influenza viruses: Human and veterinary implications. Journal of Molecular and Genetic Medicine : An International Journal of Biomedical Research. J Mol Genet Med. 3(1):158–166. 19565018 [PMC free article] [PubMed] [Google Scholar]
- Malik YS, Sircar S, Bhat S, Sharun K, Dhama K, Dadar M, Tiwari R, Chaicumpa W.. 2020. a. Emerging novel Coronavirus (2019-nCoV)-Current scenario, evolutionary perspective based on genome analysis and recent developments. Vet Q. 40(1):68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik YS, Sircar S, Bhat S, Vinodhkumar OR, Tiwari R, Sah R, Rabaan AA, Rodriguez-Morales AJ, Dhama K.. 2020. b. Emerging coronavirus disease(COVID-19), a pandemic public health emergency with animal linkages: Current status update. Indian J Anim Sci. 90(3):158–173. [Google Scholar]
- Mallapaty S. 2020. China set to clamp down permanently on wildlife trade in wake of coronavirus. Nature. doi: 10.1038/d41586-020-00499-2 [DOI] [PubMed] [Google Scholar]
- Mallapaty S. 2020. Coronavirus can infect cats - dogs, not so much. Nature. doi: 10.1038/d41586-020-00984-8 [DOI] [PubMed] [Google Scholar]
- Mihindukulasuriya KA, Wu G, St Leger J, Nordhausen RW, Wang D.. 2008. Identification of a novel coronavirus from a beluga whale by using a panviral microarray. J Virol. 82(10):5084–5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitjà O, Clotet B.. 2020. Use of antiviral drugs to reduce COVID-19 transmission. Lancet Glob Health. 8(5):e639–e640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohd HA, Al-Tawfiq JA, Memish ZA.. 2016. Middle East Respiratory Syndrome Coronavirus (MERS-CoV) origin and animal reservoir. Virol J. 13:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monchatre-Leroy E, Boue F, Boucher JM, Renault C, Moutou F, Ar Gouilh M, Umhang G.. 2017. Identification of Alpha and Beta Coronavirus in wildlife species in France: bats, rodents, rabbits, and hedgehogs. Viruses. 9(12):364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munster VJ, Feldmann F, Williamson BN, van Doremalen N, Pérez-Pérez L, Schulz J, Meade-White K, Okumura A, Callison J, Brumbaugh B, et al. 2020. Respiratory disease and virus shedding in rhesus macaques inoculated with SARS-CoV-2. Nature. doi: 10.1038/s41586-020-2324-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munjal A, Khandia R, Dhama K, Sachan S, Karthik K, Tiwari R, Malik YS, Kumar D, Singh RK, Iqbal HMN, et al. 2017. Advances in developing therapies to combat Zika virus: current knowledge and future perspectives. Front Microbiol. 8:1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch DR, French NP.. 2020. COVID-19: another infectious disease emerging at the animal-human interface. N Z Med J. 133(1510):12–15. [PubMed] [Google Scholar]
- Nishiura H, Linton NM, Akhmetzhanov AR.. 2020. Initial cluster of novel coronavirus (2019-nCoV) Infections in Wuhan, China is consistent with substantial human-to-human transmission. JCM. 9(2):488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OIE. 2020. Animal and environmental investigation to identify the zoonotic source of the COVID-19 virus. [Accessed 2020 March 3]. COVID19_21Feb.pdf.
- Parry NMA. 2020. COVID-19 and pets: when pandemic meets panic. Forensic Science International: Reports. 2:100090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A, Jernigan DB, 2019-nCoV CDC Response Team . 2020. Initial Public Health Response and Interim Clinical Guidance for the 2019 Novel Coronavirus Outbreak - United States, December 31, 2019-February 4, 2020. MMWR Morb Mortal Wkly Rep. 69(5):140–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeri NC, Shrestha N, Rahman MS, Zaki R, Tan Z, Bibi S, Baghbanzadeh M, Aghamohammadi N, Zhang W, Haque U.. 2020. The SARS, MERS and novel coronavirus (COVID-19) epidemics, the newest and biggest global health threats: what lessons have we learned?. Int J Epidemiol. doi: 10.1093/ije/dyaa033.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phadke M, Saunik S.. 2020. COVID-19 treatment by repurposing drugs until the vaccine is in sight. Drug Dev Res. doi: 10.1002/ddr.21666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadan N, Shaib H.. 2019. Middle East respiratory syndrome coronavirus (MERS-CoV): a review. Germs. 9(1):35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren LL, Wang YM, Wu ZQ, Xiang ZC, Guo L, Xu T, Jiang YZ, Xiong Y, Li YJ, Li H, et al. 2020. Identification of a novel coronavirus causing severe pneumonia in human: a descriptive study. Chin Med J (Engl). 133(9):1015–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusken CB, Farag EA, Jonges M, Godeke GJ, El-Sayed AM, Pas SD, Koopmans MP.. 2014. Middle East respiratory syndrome coronavirus (MERS-CoV) RNA and neutralising antibodies in milk collected according to local customs from dromedary camels, Qatar, April 2014. Euro Surveill. 19(23):20829. [DOI] [PubMed] [Google Scholar]
- Riccucci M. 2012. Bats as materia medica: an ethnomedical reviewe and implications for conservation. Vespertilio. 16:249–270. [Google Scholar]
- Roberts A, Lamirande EW, Vogel L, Jackson JP, Paddock CD, Guarner J, Zaki SR, Sheahan T, Baric R, Subbarao K.. 2008. Animal models and vaccines for SARS-CoV infection. Virus Res. 133(1):20–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Morales AJ, Gallego V, Escalera-Antezana JP, Mendez CA, Zambrano LI, Franco-Paredes C, Suárez JA, Rodriguez-Enciso HD, Balbin-Ramon GJ, Savio-Larriera E, et al. 2020. a. COVID-19 in Latin America: the implications of the first confirmed case in Brazil. Travel Med Infect Dis. doi: 10.1016/j.tmaid.2020.101613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Morales AJ, Bonilla-Aldana DK, Balbin-Ramon GJ, Rabaan AA, Sah R, Paniz-Mondolfi A, Pagliano P, Esposito S.. 2020. b. History is repeating itself, a probable zoonotic spillover as a cause of an epidemic: the case of 2019 novel Coronavirus. Infez Med. 28(1):3–5. [PubMed] [Google Scholar]
- Rodriguez-Morales AJ, Bonilla-Aldana DK, Tiwari R, Sah R, Rabaan AA, Dhama K.. 2020. c. COVID-19, an emerging coronavirus infection: current scenario and recent developments – an overview. J. Pure Appl. Microbiol. 14(1):6150. [Google Scholar]
- Rothan HA, Byrareddy SN.. 2020. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak [published online ahead of print, 2020 Feb 26]. J Autoimmun. 109:102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rundle AG, Park Y, Herbstman JB, Kinsey EW, Wang YC.. 2020. COVID-19 Related School Closings and Risk of Weight Gain Among Children. Obesity (Silver Spring). doi: 10.1002/oby.22813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salata C, Calistri A, Parolin C, Palu G.. 2020. Coronaviruses: a paradigm of new emerging zoonotic diseases. Pathog Dis. 77(9):ftaa006. doi: 10.1093/femspd/ftaa006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serper M, Volk ML.. 2018. Current and Future Applications of Telemedicine to Optimize the Delivery of Care in Chronic Liver Disease. Clin Gastroenterol Hepatol. 16(2):157–161.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang W, Yang Y, Rao Y, Rao X.. 2020. The outbreak of SARS-CoV-2 pneumonia calls for viral vaccines. NPJ Vaccines. 5:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Wen Z, Zhong G, Yang H, Wang C, Huang B, Liu R, He X, Shuai L, Sun Z, et al. 2020. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS-coronavirus 2. Science. 8:eabb7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh RK, Dhama K, Chakraborty S, Tiwari R, Natesan S, Khandia R, Munjal A, Vora KS, Latheef SK, Karthik K, Singh Malik Y, Singh R, et al. 2019. Nipah virus: epidemiology, pathology, immunobiology and advances in diagnosis, vaccine designing and control strategies - a comprehensive review. Vet Q. 39(1):26–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song P, Karako T.. 2020. COVID-19: Real-time dissemination of scientific information to fight a public health emergency of international concern. Biosci Trends. 14(1):1–2. [DOI] [PubMed] [Google Scholar]
- Su S, Wong G, Shi W, Liu J, Lai ACK, Zhou J, Liu W, Bi Y, Gao GF.. 2016. Epidemiology, genetic recombination, and pathogenesis of Coronaviruses. Trends Microbiol. 24(6):490–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Otake Y, Uchimoto S, Hasebe A, Goto Y.. 2020. Genomic characterization and phylogenetic classification of bovine Coronaviruses through whole genome sequence analysis. Viruses. 12(2):183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taconic Biosciences . 2020. Taconic Biosciences’ Coronavirus (COVID-19) toolkit. [Accessed 2020 April 1]. https://www.taconic.com/taconic-biosciences-coronavirus-covid-19-toolkit.html#comm.
- Tang XC, Zhang JX, Zhang SY, Wang P, Fan XH, Li LF, Li G, Dong BQ, Liu W, Cheung CL, et al. 2006. Prevalence and genetic diversity of coronaviruses in bats from China. J Virol. 80(15):7481–7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TBRI . 2020. Texas Biomedical Research Institute. Texas Biomed accelerates multi-species study to identify COVID-19 animal model. [Accessed 2020 April 1]. https://eurekalert.org/pub_releases/2020-03/tbri-tba032620.php.
- Tekes G, Thiel HJ.. 2016. Feline coronaviruses: pathogenesis of feline infectious peritonitis. Adv Virus Res. 96:193–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Department of Agriculture. 2020. USDA statement on the confirmation of COVID-19 in a tiger in New York. [Accessed 2020 April 7]. https://www.aphis.usda.gov/aphis/newsroom/news/sa_by_date/sa-2020/ny-zoo-covid-19.
- Villamil-Gómez WE, Sánchez A, Gelis L, Silvera LA, Barbosa J, Otero-Nader O, Bonilla-Salgado CD, Rodríguez-Morales AJ.. 2020. Fatal Human Coronavirus 229E (HCoV 229E) and RSV–Related Pneumonia in an AIDS patient from Colombia. Travel Med Infect Dis. :101573. doi: 10.1016/j.tmaid.2020.101573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q. 2020. hACE2 transgenic mouse model for coronavirus (covid-19) research. [Accessed 2020 April 1]. https://www.jax.org/news-and-insights/2020/february/introducing-mouse-model-for-corona-virus.
- Wang LF, Eaton BT.. 2007. Bats, civets and the emergence of SARS. Curr Top Microbiol Immunol. 315:325–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Jin X.. 2020. The progress of 2019 Novel Coronavirus (2019-nCoV) event in China. J Med Virol. 92(5):468–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Tang J, Wei F.. 2020. Updated understanding of the outbreak of 2019 novel coronavirus (2019-nCoV) in Wuhan, China. J Med Virol. 92 (4):441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassenaar TM, Zou Y.. 2020. 2019_nCoV: rapid classification of betacoronaviruses and identification of traditional Chinese medicine as potential origin of zoonotic coronaviruses. Lett Appl Microbiol. 70(5):342–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Li X, Cui J.. 2020. Evolutionary perspectives on novel Coronaviruses identified in pneumonia cases in China. Natl Sci Rev. 7(2):239–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss SR, Leibowitz JL.. 2011. Coronavirus pathogenesis. Adv Virus Res. 81:85–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo PC, Lau SK, Lam CS, Lau CC, Tsang AK, Lau JH, Bai R, Teng JL, Tsang CC, Wang M, et al. 2012. Discovery of seven novel mammalian and avian coronaviruses in the genus deltacoronavirus supports bat coronaviruses as the gene source of alphacoronavirus and betacoronavirus and avian coronaviruses as the gene source of gammacoronavirus and deltacoronavirus. J Virol. 86(7):3995–4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood C. 2020. Infections without borders: a new coronavirus in Wuhan, China. Br J Nurs. 29 (3):166–167. [DOI] [PubMed] [Google Scholar]
- Wong ACP, Li X, Lau SKP, Woo P.. 2019. Global epidemiology of bat coronaviruses. Viruses. 11(2):174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . 2020. Coronavirus disease 2019 (COVID-19) Situation Report – 103. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200502-covid-19-sitrep-103.pdf?sfvrsn=d95e76d8_4.
- WHO . 2003. World Health Organization. Consensus document on the epidemiology of severe acute respiratory syndrome (SARS). [Accessed 2020 January 29]. https://www.who.int/csr/sars/en/WHOconsensus.pdf.
- Wu A, Peng Y, Huang B, Ding X, Wang X, Niu P, Meng J, Zhu Z, Zhang Z, Wang J, et al. 2020. a. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe. 27(3):325–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F, Zhao S, Yu B, Chen YM, Wang W, Song ZG, Hu Y, Tao ZW, Tian JH, Pei YY, Yuan ML, Zhang YL, et al. 2020. b. A new coronavirus associated with human respiratory disease in China. Nature. 579(7798):265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao K, Zhai J, Feng Y, Zhou N, Zhang X, Zou JJ, Li N, Guo Y, Li X, Shen X, et al. 2020. Isolation and characterization of 2019-nCoV-like coronavirus from Malayan Pangolins. bioRxiv. doi: 10.1101/2020.02.17.951335 [DOI] [Google Scholar]
- Xu Y. 2020. Genetic diversity and potential recombination between ferret coronaviruses from European and American lineages. J Infect. 80(3):350–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y-L, Qin P, Wang B, Liu Y, Xu G-H, Peng L, Zhou J, Zhu SJ, Huang Y-W.. 2019. Broad cross-species infection of cultured cells by Bat-HKU2 –related swine acute diarrhea syndrome coronavirus and identification of its replication in murine dendritic cells in vivo highlight its potential for diverse interspecies transmission. J Virol. 93(24):e01448–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Wu Q, Zhang Z.. 2020. a. Pangolin homology associated with 2019-nCoV. bioRxiv. doi: 10.1101/2020.02.19.950253. [DOI]
- Zhai S-L, Wei W-K, Lv D-H, Xu Z-H, Chen Q-L, Sun M-F, Li F, Wang D.. 2020. Where did SARS-CoV-2 come from? Vet Rec. 186(8):254–254. [DOI] [PubMed] [Google Scholar]
- Zhang T, Wu Q, Zhang Z.. 2020. Probable Pangolin Origin of SARS-CoV-2 Associated with the COVID-19 Outbreak. Current Biology : CB. Curr Biol. 30(7):1346–1351.e2. doi: 10.1016/j.cub.2020.03.022. 32197085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Zhang H, Huang K, Yang Y, Hui X, Gao J, Jin M.. 2020. b. SARS-CoV-2 neutralizing serum antibodies in cats: a serological investigation. bioRxiv. doi: 10.1101/2020.04.01.021196 [DOI]
- Zhang XM, Herbst W, Kousoulas KG, Storz J.. 1994. Biological and genetic characterization of a hemagglutinating coronavirus isolated from a diarrhoeic child. J Med Virol. 44(2):152–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J. 2020. SARS-CoV-2: an emerging coronavirus that causes a global threat. Int J Biol Sci. 16(10):1678–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, et al. 2020. a. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 579(7798):270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P, Fan H, Lan T, Yang XL, Shi WF, Zhang W, Zhu Y, Zhang YW, Xie QM, Mani S, Zheng XS, et al. 2018. Fatal swine acute diarrhoea syndrome caused by an HKU2-related coronavirus of bat origin. Nature. 556(7700):255–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P, Yang X-L, Wang X-G, Hu B, Zhang L, Zhang W, Si H-R, Zhu Y, Li B, Huang C-L, et al. 2020. b. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 579(7798):270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]