Summary
The current severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) outbreak has been rapidly spreading worldwide, causing serious global concern. The role that animal hosts play in disease transmission is still understudied and researchers wish to find suitable animal models for fundamental research and drug discovery. In this systematic review, we aimed to compile and discuss all articles that describe experimental or natural infections with SARS‐CoV‐2, from the initial discovery of the virus in December 2019 through to October 2020. We systematically searched four databases (Scopus, PubMed, Science Direct and Web of Science). The following data were extracted from the included studies: type of infection (natural or experimental), age, sample numbers, dose, route of inoculation, viral replication, detection method, clinical symptoms and transmission. Fifty‐four studies were included, of which 34 were conducted on animal reservoirs (naturally or experimentally infected), and 20 involved models for testing vaccines and therapeutics. Our search revealed that Rousettus aegyptiacus (fruit bats), pangolins, felines, mink, ferrets and rabbits were all susceptible to SARS‐CoV‐2, while dogs were weakly susceptible and pigs, poultry, and tree shrews were not. In addition, virus replication in mice, mink, hamsters and ferrets resembled subclinical human infection, so these animals might serve as useful models for future studies to evaluate vaccines or antiviral agents and to study host‐pathogen interactions. Our review comprehensively summarized current evidence on SARS‐CoV‐2 infection in animals and their usefulness as models for studying vaccines and antiviral drugs. Our findings may direct future studies for vaccine development, antiviral drugs and therapeutic agents to manage SARS‐CoV‐2‐caused diseases.
Keywords: animal hosts, animal models, Covid‐19, SARS‐CoV‐2, therapeutic agents, vaccines
Abbreviations
- ACE2
angiotensin‐converting enzyme 2
- Covid‐19
coronavirus disease 2019
- CoVs
coronaviruses; CRISPR, clustered Regularly Interspaced Short Palindromic Repeats Repetitive; ELISA, enzyme‐linked immunosorbent assay; HCQ, hydroxychloroquine sulphate; MERS‐CoV, middle east respiratory syndrome coronavirus; MESH, medical subject headings
- NHPs
non‐human primates
- NAbs
neutralizing antibodies
- PRISMA
preferred reporting items for systematic reviews and meta‐analyses
- RBD
receptor binding domain
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- URT
upper respiratory tract
1. INTRODUCTION
The outbreak of coronavirus disease 2019 (Covid‐19) caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), has triggered a serious global public health problem and is spreading in almost all countries around the globe with 45,797,600 confirmed cases and 1,191,670 mortalities, as of 30 October 2020.1 The current outbreak of SARS‐CoV‐2 is the third outbreak of animal coronavirus transmission to humans in the past 2 decades.
SARS‐CoV‐2 belongs to the coronaviruses (CoVs), a genus in the Coronaviridae family. Most known CoVs infect animals, are associated with self‐limiting diseases and have a narrow host range.2 However, some zoonotic CoVs have a high capability to mutate, which enables the virus to cross the species barrier to infect humans, such as SARS‐CoV, middle east respiratory syndrome coronavirus (MERS‐CoV), and SARS‐CoV‐2 zoonotic viruses, which have caused severe acute respiratory diseases in humans.3, 4, 5 These zoonotic CoVs have claimed thousands of lives in the last 2 decades.4 Fortunately, compared to SARS‐CoV and MERS‐CoV, SARS‐CoV‐2 is less pathogenic; however, the concern lies in the fact that it is highly infectious and transmissible. Moreover, asymptomatic Covid‐19 patients have been shown to contribute to the transmission of the infection around the world.6, 7
Recent reports and studies of SARS‐CoV‐2 in animals have caused unnecessary fear and panic among the population, which have adversely affected the wellbeing and health of animals. Most importantly, it is unethical to cull any animals (wild or domestic) by declaring they are ‘potential’ reservoir hosts without any conclusive evidence that they can transmit SARS‐CoV‐2 to humans. Furthermore, finding the best animal models that mimic human Covid‐19 infection is vital for scientists to understand the pathogenesis of SARS‐CoV‐2 infection. In addition, animal models are critical for testing potential vaccines and therapeutic agents.8 A suitable animal model is one that can mimic human disease in terms of viral load, morbidity, clinical signs, and the immune response. Due to the urgency and demand in the current crisis, there is a pressing need to search for a good animal model to control the SARS‐CoV‐2 outbreak.9
In this systematic review, we provide a summary of the current evidence on natural and experimental infections of SARS‐CoV‐2 in animals. We compare different animals for their susceptibility, clinical signs, transmission dynamics, and immune response. We also discuss the ability of these animals to mimic Covid‐19 pathogenesis in humans, as well as their usefulness as ideal models for preclinical testing of vaccine candidates and therapeutic agents.
2. METHODOLOGY
This systematic review was performed according to the preferred reporting items for systematic reviews and meta‐analyses (PRISMA) statement.10
2.1. Eligibility criteria
We included published articles that reported infection in domestic or wild animals with serological or molecular confirmation of SARS‐CoV‐2. Inclusion criteria included: (1) investigation of the management of SARS‐CoV‐2 and (2) natural or experimentally induced SARS‐CoV‐2‐infected animal species. Exclusion criteria included (1) infection not clearly defined as SARS‐CoV‐2, (2) non‐animal models, (3) reviews, meta‐analyses, book reviews, letters, abstracts and cases and (4) non‐English reports.
2.2. Search strategy
We systematically searched four literature databases (Scopus, PubMed, Science Direct and Web of Science). All databases were searched from December 2019 to October 2020 for relevant studies on Covid‐19 infection in animals. The systematic search was performed using a group of medical subject headings terms and text words, such as: (Covid OR SARS‐CoV‐2) AND (animal OR host OR model OR ‘animal model’ OR Zoonotic OR Pets OR veterinary OR ‘domestic animals’ OR ‘wild animals’ OR ‘animal infection’ OR Mice OR monkey OR Cat OR Dog OR Hamster OR Ferret OR Non‐human primates [NHPs] OR Rabbit OR tiger OR Rodent OR Primate OR Camelidae).
2.3. Study selection
Four reviewers participated in screening and study selection, of which, three reviewers individually reviewed the identified studies (Salma Younes, Nadin Younes and Farah Shurrab), and a fourth reviewer (Gheyath K. Nasrallah) resolved any discrepancies. All studies retrieved from our initial search strategy were imported to Endnote X9 library, after which, duplicates were removed. Figure 1 illustrates the search strategy. Studies were screened in two stages. The first stage of screening involved screening of titles, abstracts, and keywords. The second stage of screening involved examining the full‐texts of all potentially relevant studies that were identified in the first stage (Figure 1). Any disagreements were resolved by discussion. Bibliographies of the included studies were checked manually for any additional eligible studies not captured in the systematic search Figure 2.
FIGURE 1.
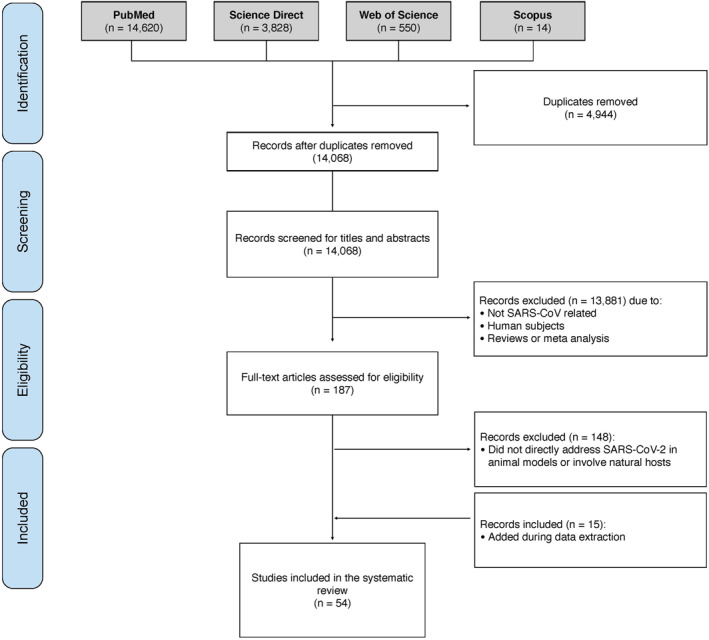
Prisma flow diagram of the systematic review study selection process. The search strategy yielded 19,012 studies. A total of 54 studies were included in the systematic review. SARS‐CoV‐2, severe acute respiratory syndrome coronavirus‐2
2.4. Data collection
Two reviewers (Salma Younes and Nadin Younes) extracted the following data from each included study: animal used, age, number of animals used, type of infection (natural/experimental), inoculation dose, route of challenge, sample, method of detection, immune response, disease and pathology (symptoms), and the possibility of animal‐animal transmission (Table S1). For vaccine and therapeutic studies on animal models infected with SARS‐CoV‐2; the following information were extracted and recorded: animal model, age, number of animals used, inoculation dose, route of challenge, vaccine/antiviral agent, dose, route of inoculation, sample type, method of detection, disease and pathology (symptoms), and the main outcomes of each study (Table S2). The information collected was reviewed independently by all authors to ensure accurate data collection.
3. RESULTS
3.1. Search findings and study characteristics
We identified 19,012 articles, of which 14,068 remained after the removal of duplicates. Thirteen thousand eight hundred eighty‐one irrelevant articles were excluded during the initial stage of screening and 148 articles were excluded during the second stage (Figure 1). The majority of the excluded studies were mainly in vivo human studies with no data on experimental or natural animal species. A total of 54 articles were included in the systematic review.
3.2. Animal species and characteristics
The studies collected using the systematic search used various animal species infected with SARS‐CoV‐2 experimentally or naturally. Most of the studies were conducted on mice (37%) and NHPs (30%), fewer studies were conducted on felines, poultry, dogs, ferrets, fruit bats, minks, hamsters, pigs, rabbits, and tree shrews as shown in Figure 2. A consolidative summary of the data gathered from all studies included in this systematic review is given in Table 1. The data collected from each study included in this systematic review are presented in Table S1 and Table S2.
FIGURE 2.
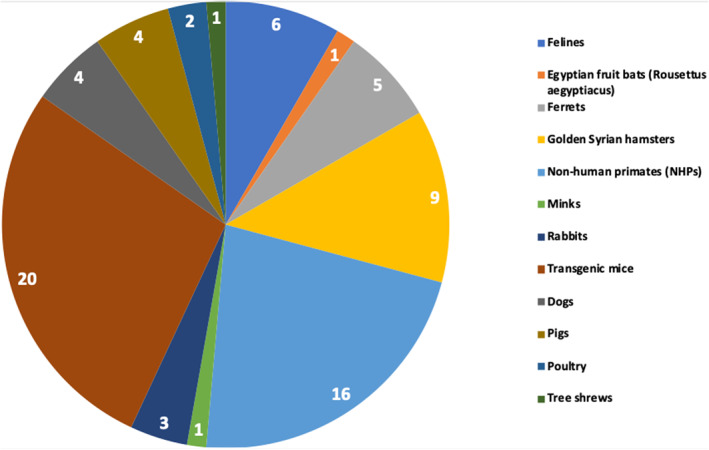
Characteristics of the included studies. Articles obtained from the systematic search were categorized based on the animal used in each study. Most of the studies were conducted on mice and NHPs. Fewer studies were conducted on felines, poultry, dogs, ferrets, fruit bats, minks, hamsters, pigs, rabbits, and tree shrews. Some studies included more than one animal; NHPs, non‐human primates
TABLE 1.
Summary of findings of SARS‐CoV‐2 infection in animals
| Animals | Type of infection (natural/experimental) | Susceptibility (high/low/none) | Clinical signs | Animal‐animal transmission (Yes/No/NR) | Immune response | Useful as a model (yes/no) | Reference |
|---|---|---|---|---|---|---|---|
| Felines | Natural and experimental | High | Yes (none to very mild in some cases) | Yes | IgG antibodies detected | No | 11, 12, 13, 14, 15, 16 |
| Egyptian fruit bats (Rousettus aegyptiacus) | Experimental | High | No | Yes | Neutralizing antibody responses | No | 17 |
| Ferrets | Experimental | High | No, (very mild in some cases) | Yes | Neutralizing antibody responses | Yes | 11, 17, 18, 19, 20 |
| Golden Syrian hamsters | Experimental | High | Yes (none to very mild in some cases) | Yes | Neutralizing antibody responses | Yes | 19, 20, 21, 22, 23, 24, 25, 26, 27, 28 |
| Non‐human primates | Experimental | High | Yes | Yes | Antibodies detected | Yes | 26, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44 |
| Minks | Natural | High | Yes | Yes | Neutralizing antibody responses | No | 13 |
| Rabbits | Experimental | High | Yes | N/A | NR | Yes | 35, 45, 46 |
| Transgenic mice | Experimental | High | Yes | Yes | Neutralizing antibody responses | Yes | 8, 14, 27, 28, 30, 32, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61 |
| Dogs | Natural and experimental | Low | No, (possible in some cases) | No | Neutralizing antibody responses | No | 11, 15, 16, 62 |
| Pigs | Experimental | None | No | No | No antibodies detected | No | 11, 17, 32, 35 |
| Poultry | Experimental | None | No | No | No antibodies detected | No | 11, 17 |
| Tree shrews | Experimental | None | Yes | NR | NR | No | 63 |
Abbreviations: NR, not reported; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus‐2.
3.3. SARS‐CoV‐2 infection in animals
There is a strong evidence that SARS‐CoV‐2 virus originated in bats64; however, the intermediate animal host is still unknown (Figure 3). Several studies showed that pangolins, snakes, turtles, and mink are all possible intermediate hosts, however, further investigations are needed.66, 67, 68 It is worth mentioning that recent findings that analyse the probable animal reservoir to Covid‐19 suggest the snake as a reservoir, based on relative synonymous codon usage bias.67 However, the missing link or intermediate link for animal to human transmission of SARS‐CoV‐2 from a recent study suggests the DNA and protein sequence of Malayan pangolins.69
FIGURE 3.
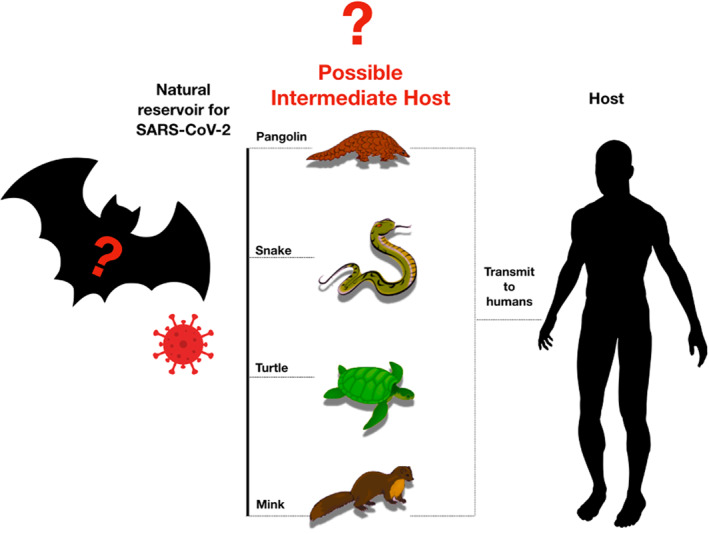
Representative figure of potential intermediate hosts. Bats are the natural reservoir origin of SARS‐CoV‐2. Pangolins are natural hosts of Betacoronaviruses. Metagenomic sequencing identified pangolin‐associated coronaviruses that belong to two sub‐lineages of SARS‐CoV‐2‐related coronaviruses65; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus‐2
Studies included in our systematic search revealed low SARS‐CoV‐2 replication in pigs, dogs, chickens, ducks, rabbits and tree‐shrews (Table 1, Table S1 and Figure 4). However, Rousettus aegyptiacus (fruit bats), pangolins, felines, mink, ferrets and rabbits were all susceptible to SARS‐CoV‐2 (Table 1, Table S1 and Figure 4). Additionally, tigers, ferrets, mink, macaques, lions, hamsters and cats are susceptible to animal‐to‐animal transmission through the airborne route (Table 1 and Table S1). Moreover, susceptible animals are capable of producing neutralizing antibodies (NAbs) that can offer protection against reinfection (Table S1); however, it remains unclear how long protection will last.
FIGURE 4.
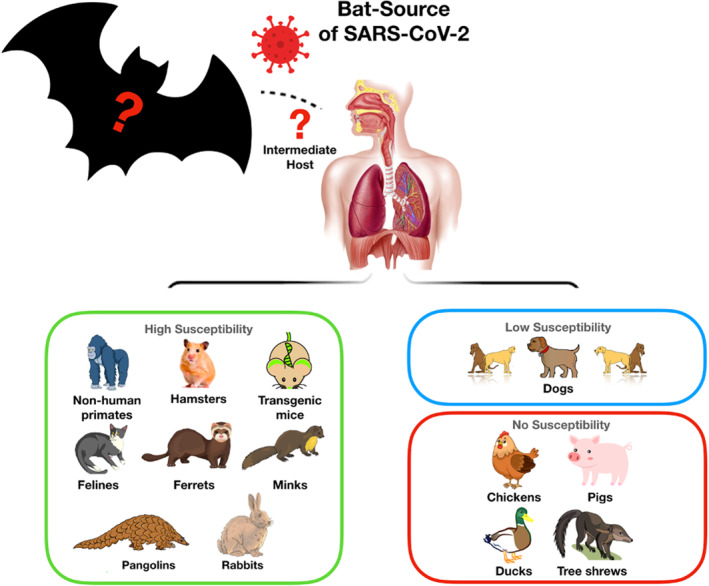
Animal host classification according to susceptibility. NHPs, hamsters, transgenic mice, felines, ferrets, minks, pangolins, and rabbits have high susceptibility, dogs have low susceptibility, while pigs, poultry, and tree shrews have no susceptibility to SARS‐CoV‐2 infection; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus‐2
4. DISCUSSION
4.1. Zoonotic links of SARS‐CoV‐2 outbreak
SARS‐CoV‐2 was first identified in Wuhan city in China in hospitalized patients who previously visited the Huanan wet seafood market where various animals including chickens, pigs, pangolins, bats, snakes, frogs, rabbits, and marmots are sold for human consumption, proposing a suitable environment for zoonotic infection spill‐over to humans.67, 70, 71, 72, 73 Scientists believe that these traditional Chinese practices might be responsible for the SARS‐CoV‐2 pandemic in humans and that the recurrent interactions between humans and animals without proper biosafety measures present a substantial risk for the occurrence of zoonotic diseases.74
Zoonotic CoVs have crossed the species barrier twice in the past 2 decades (SARS‐CoV; 2002 and MERS‐CoV; 2012). Thus, scientists have speculated that SARS‐CoV‐2 resulted from a zoonotic spillover event as well. Zoonotic CoVs need to propagate in their zoonotic reservoirs, and then, seek the chances to spillover via intermediate hosts into susceptible human targets, where they can maintain human‐to‐human transmission. Bats have been revealed as the natural hosts for several human CoVs, including HCoV‐NL63, HCoV‐229E, SARS‐CoV, and MERS‐CoV.75, 76 Genome sequence analysis confirmed that SARS‐CoV‐2 is 96% identical to the bat CoV RaTG13 at the whole‐genomic level,77 and hence bats are believed to be the primary source of origin for the novel SARS‐CoV‐2. However, the intermediate host that is yet to be elucidated.11, 67, 70, 72, 78 Researchers proposed two hypotheses for the emergence of SARS‐CoV‐2: (1) Natural selection may have occurred in an animal host before transmission to mankind; and (2) natural selection of viruses may have occurred in humans after zoonotic transmission.79 In this regard, studies involving the use of animal models or cell culture are in need to help clarify these two scenarios.79
4.2. SARS‐CoV‐2 infection in animals
Infections with SARS‐CoV‐2 have been documented in various animal species naturally or by experimental infection (Table 1 and Table S1). Our systematic search revealed that R. aegyptiacus fruit bats, pangolins, felines, minks, NHPs, hamsters, ferrets and rabbits are all susceptible to SARS‐CoV‐2, dogs are weakly susceptible, whereas pigs, poultry, and tree shrews are not susceptible to SARS‐CoV‐2 infection (Table 1 and Table S1).
4.2.1. Animals that are susceptible to SARS‐CoV‐2
Bats
SARS‐CoV and MERS‐CoV originated in bats and were capable of infecting humans.80, 81 Bats are known to be the ideal hosts for pathogenic CoVs, as viruses remain persistent in asymptomatic bats.82 Accordingly, researchers predicted the role of bats as the origin of the current SARS‐CoV‐2 outbreak.68, 82, 83, 84 Several studies showed that SARS‐CoV‐2 is virtually identical to the bat CoV on a genomic level, hence it is assumed that bats are the primary source of the SARS‐CoV‐2 spillover.83, 85 Experimental studies revealed that R. aegyptiacus fruit bats are susceptible to SARS‐CoV‐2, as detected by RT‐qPCR, immunohistochemistry, and in situ hybridisation in the nasal cavity of intranasally SARS‐CoV‐2‐inoculated fruit bats86 (Table 1 and Table S1).
Pangolins
Several studies showed evidence based on metagenomic sequencing analysis that pangolins (Manis javanica), a group of endangered mammals, may harbour the ancestral beta‐CoVs, which is the second closest relative SARS‐CoV‐2 virus.29, 65, 69 This CoV identified in pangolin share 85%–92% of their genetic sequence with SARS‐CoV‐2 virus and 90% identity with bat CoV RaTG13 virus. Thus, the pangolin novel CoV cluster into two sub‐lineages of SARS‐CoV‐2‐like viruses in the phylogenetic tree; of which one shares 97.4% amino acid sequence of the receptor binding domain (RBD) with SARS‐CoV‐2.65 Until now, the pangolin is probably one of the intermediate hosts of SARS‐CoV‐2.65
Felines
There have been several reports of human‐to‐feline transmission of SARS‐CoV‐2 (Table 1 and Table S1). Cats from Belgium and Hong Kong tested SARS‐CoV‐2‐ positive.87 Cats were shown to be susceptible to SARS‐CoV‐2 and that they may transmit the virus to other cats in close‐contact.88 However, none of the infected cats showed symptoms of SARS‐CoV‐2 disease.11, 89 Recent studies reported that cats develop NAbs against SARS‐CoV‐2, indicating that they can get the infection under natural circumstances and develop an immunological response.12 Furthermore, a higher titre of NAbs was observed among cats in close contact with infected owners.12 Nevertheless, the extremely low putative infection rates in domestic cats suggest that they are poor hosts for the SARS‐CoV‐2 virus and that the virus is unlikely to become established in wild cats in natural conditions. Other felines, such as tigers and lions have been found to test positive for Covid‐19 after interaction with SARS‐CoV‐2‐infected workers in a Zoo in New York City, USA90 (Table 1 and Table S1).
Mink
To date, there has been one report of mink infection with SARS‐CoV‐2.13 Minks in two mink farms in Beek en Donk and in Milheeze in Netherlands developed signs of breathing and gastrointestinal issues back in April 2020, with a mortality of 1.2% to 2.4%, and deaths mainly observed in pregnant females. Most of the mink showed lung lesions, including interstitial pneumonia.13 Investigations later revealed that workers at the farm had previously tested positive for Covid‐19, suggestive of human‐to‐animal transmission (Table 1 and Table S1).
Non‐human primates
It has been shown that all NHPs are susceptible to previous SARS‐CoV infection where symptoms of fever, diarrhoea and pneumonitis were reported.91, 92 Recent studies have shown that rhesus macaques are susceptible to SARS‐CoV‐2 infection. The virus was found to cause mild pneumonia similar to that in humans.30 Additionally, infected rhesus macaques developed NAbs that may protect them from subsequent infections.30 Other NHPs, such as cynomolgus macaques and common marmosets were shown to be susceptible to SARS‐CoV‐2 infection29 (Table 1 and Table S1). However, comparing the susceptibility of rhesus macaques to cynomolgus macaques and common marmosets, the former showed higher susceptibility to SARS‐CoV‐2 infection. It is worth mentioning that viral replication of nasopharyngeal swabs, anal swabs and lung in old monkeys was more active than that in young monkeys for 14 days after SARS‐CoV‐2 challenge.31 In addition, old monkeys exhibited diffuse severe interstitial pneumonia compared to young monkeys. Interestingly, a notably higher percentage of lymphocytes was observed in younger monkeys compared to older monkeys, but the decreased counts of lymphocytes were found in both age groups.31
Hamsters
Several studies have shown that hamsters are susceptible to SARS‐CoV‐2 infection (Table 1 and Table S1). Golden Syrian hamsters infected with SARS‐CoV‐2 developed clinical signs, such as lethargy, ruffled fur, hunched back, abnormal breathing and weight loss.21, 22, 23 In addition, viral transmission to naïve hamsters through direct contact was described.22 Of particular interest is the fact that infection with SARS‐CoV‐2 in hamsters reflects some of the demographic differences of Covid‐19 in humans. Thus, aged hamsters and male hamsters seem to develop more severe disease than young and female hamsters, respectively.24
Ferrets
Ferrets have been described as SARS‐CoV‐2‐susceptible in several studies (Table 1 and Table S1). The virus was shown to replicate efficiently in the upper respiratory tract.11 Infected ferrets showed high body temperature and high virus in nasal turbinate, trachea and lungs tissue, accompanied by acute bronchiolitis present in the lungs. Additionally, SARS‐CoV‐2 was detected in saliva and nasal washes of infected ferrets. Furthermore, the virus was transmitted to naïve ferrets that were in close contact with the SARS‐CoV‐2‐infected ferrets.93
Rabbits
A recent study showed that SARS‐CoV‐2 can infect rabbits, and thus they may serve as potential sources of animal to human transmission (Table 1 and Table S1).45 Nevertheless, whether or not rabbits are naturally susceptible to the virus remains under investigation.
4.2.2. Animals that are weakly/not susceptible to SARS‐CoV‐2
Dogs
Under natural conditions, there have been few reports of dogs that have tested SARS‐CoV‐2‐positive, of which none reported that dogs could pose as a source of infection for humans (Table 1 and Table S1). So far, studies conducted on dogs that have tested SARS‐CoV‐2‐positive showed that they mostly became infected after close contact with people infected with the virus. In a study conducted in Hong Kong that comprised 15 dogs from households with confirmed SARS‐CoV‐2 cases, only two out of the 15 dogs tested SARS‐CoV‐2‐positive. Both dogs demonstrated antibody responses against SARS‐CoV‐2.62 In experimental infection studies, dogs failed to support viral replication and had low susceptibility to the virus,11 and thus, failed to show potential for serving as animal models of SARS‐CoV‐2 infection (Table 1 and Table S1).
Pigs
A recent study was conducted to investigate whether pigs are susceptible to SARS‐CoV‐2 or not (Table 1 and Table S1). Swabs were found to be free from viral RNA, in addition, no viral RNA was detected in any of the naïve animals in contact, indicating that pigs could not transmit the virus. Pigs used for experimental research, inoculated intravenously, intranasally, ocularly or orally with SARS‐CoV‐2, failed to develop clinical signs. Furthermore, testing sera collected from pigs by enzyme‐linked immunosorbent assay (ELISA) on the 14th day post inoculation (dpi) showed that they were seronegative,11 which indicates that pigs did not develop any immune response to the virus. Thus, pigs are unlikely to transmit SARS‐CoV‐2 to humans and are not suitable models for studying SARS‐CoV‐2 infection. These findings are not surprising as previous studies on SARS‐CoV outbreak in 2003 these animals did not play a role as amplifying hosts for SARS‐CoV.94
Poultry
Recent evidence suggests that poultry are not susceptible to SARS‐CoV‐2 infection11 (Table 1 and Table S1). Viral RNA was not detected in swabs taken from SARS‐CoV‐2‐inoculated chickens and ducks. In addition, viral RNA was not detected in any of the naïve animals that were in close contact, indicating no animal‐to‐animal transmission. Furthermore, the inoculated chickens and ducks were found to be seronegative when tested by ELISA.11
Tree shrews
Experimental infection of tree shrews with SARS‐CoV‐2 has been described63 (Table 1 and Table S1). No clinical signs were observed in SARS‐CoV‐2‐inoculated tree shrews except high body temperature,63 and thus, may not be a suitable animal for Covid‐19 research.
4.2.3. Potential animal models that are still under research
Other animals are still under investigation whether or not they are susceptible to SARS‐CoV‐2 infection and if they can be useful as models. In Silico Analysis revealed that wild felidae, goats, spotted hyenas and civets may be SARS‐CoV‐2‐susceptible and could potentially act as intermediate hosts to transmit the virus from bats to humans.95 These findings highlight the usefulness of these animals to be used as experimental animal models for studying SARS‐CoV‐2 infection directly without the need for transgenic operation.95 It is noteworthy to mention that rodents, birds, and reptiles are unlikely to be susceptible to SARS‐CoV‐2,95 however, further investigation is needed.
4.3. Potential animal models to study vaccine and therapeutic candidates for SARS‐CoV‐2 infection
Not every susceptible animal can serve as a good model for testing vaccine and therapeutic candidates. A good animal model is defined by its ability to reproduce relevant human physiology to be used for investigating the effectiveness of potential therapeutic agents and pharmacological drugs. Thus, the identification of reliable and feasible animal models that can mimic Covid‐19 clinical symptoms of humans are urgently needed to decipher the routes of transmission and pathophysiology of the disease. Table S2 summarize the animal models that were utilized for testing vaccines and therapeutic candidates for SARS‐CoV‐2.
4.3.1. Transgenic mice
Mouse models are known to be convenient models to study a disease because they are cheap, available and easy to manipulate. However, these models are challenging to study SARS‐CoV‐2 due to differences in the usage pattern of angiotensin‐converting enzyme 2 (ACE2) receptor compared to human,96 which makes them non‐susceptible to the virus, and if they do get infected, it is at a very low rate with poor viral replication. That is because the spike protein of the virus does not recognize the ACE2 receptor of the mouse. However, transgenic mice having human ACE2 (hACE2) receptor supports SARS‐CoV‐2 infection. Several studies utilizing humanized mouse strains expressing hACE2 have been reported14, 47, 48, 97 (Table S1 and Table S2). Most of which revealed that hACE2 transgenic mice infected with SARS‐CoV‐2 they can successfully mimic human COVI‐19 symptoms.14, 47, 48 In addition, a study that utilized clustered regularly interspaced short palindromic repeats repetitive/Cas9 knocking technology to generate humanized mouse strains expressing hACE2 revealed that hACE2 mice supported the replication of SARS‐CoV‐2 in pulmonary Clara cells and macrophages,49 and they showed symptoms that are similar to those in Covid‐19 patients.49 Furthermore, infecting young adult and aged BALB/c mice adapted SARS‐CoV‐2 supported virus replication in both upper and lower airway, with severe symptoms in the aged mice.48 Similarly, SARS‐CoV‐2 infection in transducing BALB/c mice encoding hACE2 caused lung pathology, weight loss, and viral pneumonia, where high levels of viral RNA were accumulated in the lungs.98 In addition, infecting C57BL/6J (B6J) mice encoding hACE2 with SARS‐CoV‐2 caused pulmonary infiltrates which is similar to Covid‐19 patients.99
4.3.2. Ferrets
Ferrets have been widely used to study viral respiratory diseases in humans and remain one of the best candidates as an animal model for respiratory tract infections as they display most of the human‐like symptoms.71 Unlike mice, ferrets can be infected with the virus without prior transgenic adaptation. Ferrets were used as animal models for evaluating the effectiveness of antiviral drugs against influenza,100 SARS‐CoV101 and Ebola.102 Thus, researchers believe that ferrets may help in the race for finding the best therapeutic drugs and vaccines against SARS‐CoV‐2. A recent study that investigated the efficacy of different antivirals agents using infected ferrets, has revealed a reduction in the overall clinical scores following treatment with three antiviral agents; lopinavir‐ritonavir, hydroxychloroquine sulphate, and emtricitabine‐tenofovir18 (Table S2).
4.3.3. Hamsters
The Syrian hamster is another important animal model to investigate SARS‐CoV‐2 pathogenesis and transmission. The clinical features, histopathological changes, and immune responses reported in infected Syrian hamsters closely mimic those in human Covid‐19 patients.23 High replication of the virus was evident in the nasal mucosa and lower respiratory epithelial cells. Interestingly, SARS‐CoV‐2 was observed in the olfactory sensory neurons which parallels the anosmia recorded in Covid‐19 patients.21, 22, 23 NAbs were produced in recovered hamsters, and they had protection against subsequent SARS‐CoV‐2 infection.21, 22, 23 Moreover, passive transfer of serum to naive hamsters was found to efficiently suppress SARS‐CoV‐2 replication in lung tissue21 and to provide protection against high dose challenge of the SARS‐CoV‐2 virus,25 this occurred without any significant histopathological changes or clinical signs.23 Thus, Syrian hamsters are potentially good candidates as a small model for evaluating the efficacy of vaccines and antiviral drugs.21 In a study investigating the effect of hydroxychloroquine in hamsters,26 no significant difference between infected and control hamsters was observed, indicating that this drug does not inhibit either viral replication or shedding, or further, reduce the clinical symptoms of the disease in hamsters (Table S2).
4.3.4. Non‐human primates
NHPs are considered as one of the best animal models to investigate potential antiviral drugs, therapeutic agents, and to gain a better understanding of the aspects related to pathology in different infectious diseases.103 Most of the current knowledge about SARS‐CoV‐2 infection in NHPs is currently preprints, repositories, and inoculation studies. Interestingly, not all infected NHPs showed symptoms of the infection, even though the virus was detected in the nasal swab collections.104 These findings resemble the asymptomatic patients, who still can participate in viral transmission without showing any symptoms.105 Rosenke and his colleagues tested the effect of hydroxychloroquine on 10 Rhesus macaques infected with SARS‐CoV‐2. Similar to their observations in the hamster model, this drug showed no significant prophylactic or therapeutic benefit in the infected rhesus macaque, similar to humans, despite its promising effects in vitro. Further, six studies were also conducted to test potential therapeutic vaccines and antiviral drugs on NHPs; pigtail macaques,32 Rhesus macaque,26, 33, 34, 35, 36 and cynomolgus macaques35 as shown in Table S2. All included studies indicated that NHPs are considered good indicators and preclinical models for testing drugs prior to moving to clinical trials (Table S2).
4.3.5. Other animals
Other animals that have been used for vaccine studies in Covid‐19 research include pigs and rabbits (Table S2). However, there is a lack of studies on these animals, and thus, further investigation on the usefulness of these animals as models is needed.
5. LIMITATIONS
This review has several limitations. First, few studies are currently available for inclusion. Second, more detailed information on the collection and sample animals, particularly regarding their clinical findings and conditions during collection, was unavailable in most studies at the time of conducting the review.
6. CONCLUSIONS AND FUTURE PERSPECTIVES
This is the first systematic review conducted to summarize current evidence on SARS‐CoV‐2 infection in domestic and wild animals. In summary, SARS‐CoV‐2 has a zoonotic origin and was transmitted to humans via an undetermined intermediate host, leading to infections in humans and other mammals. To enter host cells, the viral spike protein binds to its receptor, ACE2, and is then processed by TMPRSS2. Based on our systematic review, we suggest that SARS‐CoV‐2 can infect a broad range of mammals, but few fish, birds or reptiles. For instance, R. aegyptiacus fruit bats, pangolins, felines, minks, NHPs, hamsters, ferrets, and rabbits are all susceptible to SARS‐CoV‐2, and dogs are weakly susceptible. While poultry and pigs, and tree shrews are not susceptible to SARS‐CoV‐2 infection. Mice, mink, hamsters, and ferrets developed clinical signs of the infection that are very similar to that in humans. Thus, these animals might serve as robust useful small‐animal models for future studies to test the efficacy of vaccines and antiviral agents. Whilst, cats, hamsters and ferrets may serve as a useful model for studying the virus transmission and the efficacy of antiviral drugs to limit the spread of the infection. Most importantly, the NHPs were demonstrated to be the most relevant animal model to assess the antiviral agents and vaccine effectiveness before rapid deployment to clinical trials on humans. Although there are more than 180 vaccine candidates and more than 23 well‐known pharmaceutical companies that are trying to produce an effective vaccine against SARS‐CoV‐2, until now, no vaccines were finally approved for human use. However, the current literature data so far proposed that safe vaccines could be available within the coming few months. For more information about vaccine development, the readers are advised to refer back to this comprehensive review and website.106, 107 It is clear now that among all virus proteins, the most antigenic target for successful active or passive vaccination against all coronavirus, including SARS‐CoV‐2, is the S1 subunit of the spike S proteins; more specifically, the RBDs of the S1 subunit. That is, if a vaccinated host produces NAbs to the RBD, it will block virus binding and, consequently, viral replication. In fact, it was shown that passive vaccination with a cocktail of humanized monoclonal antibodies targeting the RBD has shown effectiveness in the treatment of Covid‐19 patients.108 The RBD viral antigen could be injected in a form of self‐replicating mRNA or a recombinant protein. Different delivery systems are currently being used in clinical trials for delivering the virus RNA or the recombinant protein such as the lipid nanoparticles or the non‐pathogenic adenovirus associated vector. Moreover, other companies are also trying to produce live attenuated (a genetically weakened version of the virus) or chemically inactivated whole virus vaccine.106 Finally, our systematic search revealed that viral shedding from pets is not sufficient to infect other members of the family or other animals. However, susceptible animals could serve as reservoirs of the virus, necessitating careful ongoing animal management and surveillance.
CONFLICT OF INTERESTS
The authors declare that they have no competing interests.
AUTHOR CONTRIBUTIONS
Conceptualization, Gheyath K. Nasrallah; data curation, Salma Younes, Nadin Younes, Farah Shurrab and Gheyath K. Nasrallah; writing—original draft preparation, Salma Younes, Nadin Younes, Farah Shurrab and Gheyath K. Nasrallah; writing—review and editing, Salma Younes, Nadin Younes, Farah Shurrab and Gheyath K. Nasrallah; visualization, Salma Younes, Nadin Younes, Farah Shurrab and Gheyath K. Nasrallah; supervision, Gheyath K. Nasrallah; project administration, Gheyath K. Nasrallah. All authors have read and agreed to the published version of the manuscript.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
Open access funding provided by the Qatar National Library. This report was made possible by an RRC award [RRC‐2‐032] from the Qatar National Research Fund (a member of The Qatar Foundation). The statements made herein are solely the responsibility of the authors.
Younes S, Younes N, Shurrab F, Nasrallah GK. Severe acute respiratory syndrome coronavirus‐2 natural animal reservoirs and experimental models: systematic review. Rev Med Virol. 2021;31(4):e2196. 10.1002/rmv.2196
References
REFERENCES
- 1. WHO . WHO Coronavirus disease (COVID‐19) [Online; WHO; cited 28 Jun 2020]. 2020. Available from https://covid19.who.int. [Google Scholar]
- 2. Liu DX, Liang JQ, Fung TS. Human coronavirus‐229E, ‐OC43, ‐NL63, and ‐HKU1. Life Sci. 2020. B978‐0‐12‐809633‐8.21501‐X. [Google Scholar]
- 3. da Silva PG, Mesquita JR, de São José Nascimento M, Ferreira VAM. Viral, host and environmental factors that favor anthropozoonotic spillover of coronaviruses: an opinionated review, focusing on SARS‐CoV, MERS‐CoV and SARS‐CoV‐2. Sci Total Environ. 2021;750:141483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Decaro N, Lorusso A. Novel human coronavirus (SARS‐CoV‐2): a lesson from animal coronaviruses. Vet Microbiol. 2020;244:108693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bizzotto J, Sanchis P, Abbate M, et al. SARS‐CoV‐2 infection boosts MX1 antiviral effector in COVID‐19 patients. iScience. 2020;23(10):101585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Al‐Sadeq DW, Nasrallah GK. The incidence of the novel coronavirus SARS‐CoV‐2 among asymptomatic patients: a systematic review. Int J Infect Dis. 2020;98:372–380. 10.1016/j.ijid.2020.06.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Younes N, Al‐Sadeq DW, Al‐Jighefee H, et al. Challenges in laboratory diagnosis of the novel coronavirus SARS‐CoV‐2. Viruses. 2020;12(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sun J, Zhuang Z, Zheng J, et al. Generation of a broadly useful model for COVID‐19 pathogenesis, vaccination, and treatment. Cell. 2020;182(3):734–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Misra DP, Agarwal V, Gasparyan AY, Zimba O. Rheumatologists' perspective on coronavirus disease 19 (COVID‐19) and potential therapeutic targets. Clin Rheumatol. 2020;39(7):2055‐2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moher D, Liberati A, Tetzlaff J, Altman DG, The PG. Preferred reporting Items for systematic reviews and meta‐analyses: the PRISMA statement. PLOS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shi J, Wen Z, Zhong G, et al. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS‐coronavirus 2. Science. 2020;368(6494). 1016‐1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang Q, Zhang H, Huang K, et al. SARS‐CoV‐2 neutralizing serum antibodies in cats: a serological investigation. bioRxiv. 2020. 10.1101/2020.04.01.021196. [DOI] [Google Scholar]
- 13. Oreshkova N, Molenaar RJ, Vreman S, et al. SARS‐CoV‐2 infection in farmed minks, the Netherlands, April and May 2020. Eurosurveillance. 2020;25(23):2001‐2005. 10.2807/1560-7917.es.2020.25.23.2001005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bao L, Deng W, Huang B, et al. The pathogenicity of SARS‐CoV‐2 in hACE2 transgenic mice. Nature. 2020;583 (7818):830–833. 10.1038/s41586-020-2312-y. [DOI] [PubMed] [Google Scholar]
- 15. Sailleau C, Dumarest M, Vanhomwegen J, et al. First detection and genome sequencing of SARS‐CoV‐2 in an infected cat in France. Transbound Emerg Dis. 2020. 10.1111/tbed.13659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Temmam S, Barbarino A, Maso D, et al. Absence of SARS‐CoV‐2 infection in cats and dogs in close contact with a cluster of COVID‐19 patients in a veterinary campus. One Health. 2020;10:100164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schlottau K, Rissmann M, Graaf A, et al. Experimental transmission studies of SARS‐CoV‐2 in fruit bats, ferrets, pigs and chickens. SSRN Electron J. 2020;1(5):218–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Park SJ, Yu KM, Kim YI, et al. Antiviral efficacies of FDA‐approved drugs against SARS‐CoV‐2 infection in ferrets. Mbio. 2020;11(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Richard M, Kok A, de Meulder D, et al. SARS‐CoV‐2 is transmitted via contact and via the air between ferrets. Nat Commun. 2020;11(1):3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Blanco‐Melo D, Nilsson‐Payant BE, Liu WC, et al. Imbalanced host response to SARS‐CoV‐2 drives development of COVID‐19. Cell. 2020;181(5):1036‐1045 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Imai M, Iwatsuki‐Horimoto K, Hatta M, et al. Syrian hamsters as a small animal model for SARS‐CoV‐2 infection and countermeasure development. In: Proceedings of the National Academy of Sciences of the United States of America. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sia SF, Yan LM, Chin AWH, et al. Pathogenesis and transmission of SARS‐CoV‐2 in golden hamsters. Nature. 2020;583(7818):834–838. 10.1038/s41586-020-2342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chan JF, Zhang AJ, Yuan S, et al. Simulation of the clinical and pathological manifestations of coronavirus Disease 2019 (COVID‐19) in a golden Syrian hamster model: implications for disease pathogenesis and transmissibility. Clin Inf Dis. 2020. 10.1093/cid/ciaa325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Osterrieder N, Bertzbach LD, Dietert K, et al. Age‐dependent progression of SARS‐CoV‐2 infection in Syrian hamsters. Viruses. 2020;12(7):779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rogers TF, Zhao F, Huang D, et al. Rapid isolation of potent SARS‐CoV‐2 neutralizing antibodies and protection in a small animal model. bioRxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rosenke K, Jarvis MA, Feldmann F, et al. Hydroxychloroquine proves ineffective in hamsters and macaques infected with SARS‐CoV‐2. bioRxiv. 2020. [Google Scholar]
- 27. Boudewijns R, Thibaut HJ, Kaptein SJF, et al. STAT2 signaling as double‐edged sword restricting viral dissemination but driving severe pneumonia in SARS‐CoV‐2 infected hamsters. bioRxiv. 2020. 10.1101/2020.04.23.056838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li W, Schäfer A, Kulkarni SS, et al. High potency of a bivalent human VH domain in SARS‐CoV‐2 animal models. Cell. 2020;183(2). 429‐441 e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lu S, Zhao Y, Yu W, et al. Comparison of SARS‐CoV‐2 infections among 3 species of non‐human primates. bioRxiv. 2020. [Google Scholar]
- 30. Bao L, Deng W, Gao H, et al. Reinfection could not occur in SARS‐CoV‐2 infected rhesus macaques. bioRxiv. 2020. 10.1101/2020.03.13.990226. [DOI] [Google Scholar]
- 31. Yu P, Qi F, Xu Y, et al. Age‐related rhesus macaque models of COVID‐19. Animal Models and Experimental Medicine. 2020;3(1):93‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Erasmus JH, Khandhar AP, Walls AC, et al. Single‐dose replicating RNA vaccine induces neutralizing antibodies against SARS‐CoV‐2 in nonhuman primates. bioRxiv. 2020. [Google Scholar]
- 33. van Doremalen N, Lambe T, Spencer A, et al. ChAdOx1 nCoV‐19 vaccination prevents SARS‐CoV‐2 pneumonia in rhesus macaques. bioRxiv. 2020. [Google Scholar]
- 34. Williamson BN, Feldmann F, Schwarz B, et al. Clinical benefit of remdesivir in rhesus macaques infected with SARS‐CoV‐2. Nature. 2020;585(7824):273–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang H, Zhang Y, Huang B, et al. Development of an inactivated vaccine candidate, BBIBP‐CorV, with potent protection against SARS‐CoV‐2. Cell. 2020;6(182):713–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yu J, Tostanoski LH, Peter L, et al. DNA vaccine protection against SARS‐CoV‐2 in rhesus macaques. Science. 2020;369(6505):806–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chandrashekar A, Liu J, Martinot AJ, et al. SARS‐CoV‐2 infection protects against rechallenge in rhesus macaques. Science. 2020;369(6505):812–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Finch CL, Crozier I, Lee JH, et al. Characteristic and quantifiable COVID‐19‐like abnormalities in CT‐ and PET/CT‐imaged lungs of SARS‐CoV‐2‐infected crab‐eating macaques (Macaca fascicularis). bioRxiv. 2020. [Google Scholar]
- 39. Halfmann PJ, Hatta M, Chiba S, et al. Transmission of SARS‐CoV‐2 in domestic cats. N Engl J Med. 2020;383(6):592–594. 10.1056/nejmc2013400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Munster VJ, Feldmann F, Williamson BN, et al. Respiratory disease and virus shedding in rhesus macaques inoculated with SARS‐CoV‐2. bioRxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Woolsey C, Borisevich V, Prasad AN, et al. Establishment of an African green monkey model for COVID‐19. bioRxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gao Q, Bao L, Mao H, et al. Rapid development of an inactivated vaccine candidate for SARS‐CoV‐2. Science. 2020:eabc1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Deng W, Bao L, Gao H, et al. Ocular conjunctival inoculation of SARS‐CoV‐2 can cause mild COVID‐19 in rhesus macaques. Nat Commun. 2020;11(1):4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Aid M, Busman‐Sahay K, Vidal SJ, et al. Vascular disease and thrombosis in SARS‐CoV‐2‐infected Rhesus Macaques. Cell; 2020;8674(20):31311–31318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mykytyn AZ, Lamers MM, Okba NMA, et al. Susceptibility of rabbits to SARS‐CoV‐2. bioRxiv. 2020. 10.1101/2020.08.27.263988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ravichandran S, Coyle EM, Klenow L, et al. Antibody signature induced by SARS‐CoV‐2 spike protein immunogens in rabbits. Sci Transl Med. 2020;12(550):eabc3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jiang RD, Liu MQ, Chen Y, et al. Pathogenesis of sars‐cov‐2 in transgenic mice expressing human angiotensin‐converting enzyme 2. Cell. 2020;182(1):50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dinnon KH, Leist SR, Schäfer A, et al. A mouse‐adapted SARS‐CoV‐2 model for the evaluation of COVID‐19 medical countermeasures. BioRxiv. 2020. [Google Scholar]
- 49. Sun SH, Chen Q, Gu HJ, et al. A mouse model of SARS‐CoV‐2 infection and pathogenesis. Cell Host Microbe. 2020;28(1):124–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Israelow B, Song E, Mao T, et al. Mouse model of SARS‐CoV‐2 reveals inflammatory role of type I interferon signaling. bioRxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pruijssers AJ, George AS, Schäfer A, et al. Remdesivir potently inhibits SARS‐CoV‐2 in human lung cells and chimeric SARS‐CoV expressing the SARS‐CoV‐2 RNA polymerase in mice. bioRxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sheahan TP, Sims AC, Zhou ST, et al. An orally bioavailable broad‐spectrum antiviral inhibits SARS‐CoV‐2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Sci Transl Med. 2020;12(541):5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wu Y, Wang F, Shen C, et al. A noncompeting pair of human neutralizing antibodies block COVID‐19 virus binding to its receptor ACE2. Science. 2020;368(6496):1274‐1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Xia S, Liu M, Wang C, et al. Inhibition of SARS‐CoV‐2 (previously 2019‐nCoV) infection by a highly potent pan‐coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell Res. 2020;30(4):343‐355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yi C, Sun X, Ye J, et al. Key residues of the receptor binding motif in the spike protein of SARS‐CoV‐2 that interact with ACE2 and neutralizing antibodies. Cell Mol Immunol. 2020;17(6):621–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zost SJ, Gilchuk P, Case JB, et al. Potently neutralizing human antibodies that block SARS‐CoV‐2 receptor binding and protect animals. bioRxiv. 2020. [Google Scholar]
- 57. Li W, Drelich A, Martinez DR, et al. Potent neutralization of SARS‐CoV‐2 in vitro and in an animal model by a human monoclonal antibody. bioRxiv. 2020. 10.1101/2020.05.13.093088. [DOI] [Google Scholar]
- 58. Gu H, Chen Q, Yang G, et al. Rapid adaptation of SARS‐CoV‐2 in BALB/c mice: novel mouse model for vaccine efficacy. bioRxiv. 2020. [Google Scholar]
- 59. Lv H, Wu NC, Tsang OT‐Y, et al. Cross‐reactive antibody response between SARS‐CoV‐2 and SARS‐CoV infections. bioRxiv. 2020. 10.1101/2020.03.15.993097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Leist SR, Dinnon KH, Schäfer A, et al. A mouse‐adapted SARS‐CoV‐2 induces acute lung injury and mortality in standard laboratory mice. Cell. 2020;8674(20):31247. 10.1016/j.cell.2020.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hanifehnezhad A, Kehribar EŞ, Öztop S, et al. Characterization of local SARS‐CoV‐2 isolates and pathogenicity in IFNAR−/‐ mice. Heliyon. 2020;6(9):e05116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sit THC, Brackman CJ, Ip SM, et al. Infection of dogs with SARS‐CoV‐2. Nature. 2020;586(7831):776–778. 10.1038/s41586-020-2334-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zhao Y, Wang J, Kuang D, et al. Susceptibility of tree shrew to SARS‐CoV‐2 infection. BioRxiv. 2020. 10.1101/2020.04.30.029736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Letko M, Seifert SN, Olival KJ, Plowright RK, Munster VJ. Bat‐borne virus diversity, spillover and emergence. Nat Rev Microbiol. 2020;18(8):461‐471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lam TTY, Jia N, Zhang YW, et al. Identifying SARS‐CoV‐2‐related coronaviruses in Malayan pangolins. Nature. 2020;583(7815):282–285. 10.1038/s41586-020-2169-0. [DOI] [PubMed] [Google Scholar]
- 66. Yuan S, Jiang S‐C, Li Z‐L. Analysis of possible intermediate hosts of the new coronavirus SARS‐CoV‐2. Front Vet Sci. 2020;7:379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ji W, Wang W, Zhao X, Zai J, Li X. Cross‐species transmission of the newly identified coronavirus 2019‐nCoV. J Med Virol. 2020;92(4):433‐440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zhao J, Cui W, Tian B‐p. The potential intermediate hosts for SARS‐CoV‐2. Front Microbiol. 2020;11:2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zhang T, Wu Q, Zhang Z. Probable pangolin origin of SARS‐CoV‐2 associated with the COVID‐19 outbreak. Curr Biol. 2020;30(7):1346‐1351 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hui DS, I Azhar E, Madani TA, et al. The continuing 2019‐nCoV epidemic threat of novel coronaviruses to global health—the latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2020;91:264‐266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Liu ZX, Xiao X, Wei XL, et al. Composition and divergence of coronavirus spike proteins and host ACE2 receptors predict potential intermediate hosts of SARS‐CoV‐2. J Med Virol. 2020;92(6):595‐601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Okba NMA, Müller MA, Li W, et al. Severe acute respiratory syndrome coronavirus 2‐specific antibody responses in coronavirus disease patients. Emerg Infect Dis. 2020;26(7):1478‐1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Salata C, Calistri A, Parolin C, Palù G. Coronaviruses: A paradigm of new emerging zoonotic diseases. Pathog Dis. 2020;77(9):006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Cui J, Li F, Shi ZL. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019;17(3):181‐192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zhou P, Yang X‐L, Wang X‐G, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Nishiura H, Linton N, Akhmetzhanov A. Initial cluster of novel coronavirus (2019‐nCoV) infections in Wuhan, China is consistent with substantial human‐to‐human transmission. J Clin Med. 2020;9(2):419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Andersen KG, Rambaut A, Lipkin WI, Holmes EC, Garry RF. The proximal origin of SARS‐CoV‐2. Nat Med. 2020;26(4):450‐452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wang L‐F, Shi Z, Zhang S, Field H, Daszak P, Eaton BT. Review of bats and SARS. Emerg Infect Dis. 2006;12(12):1834‐1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lu G, Wang Q, Gao GF. Bat‐to‐human: spike features determining 'host jump' of coronaviruses SARS‐CoV, MERS‐CoV, and beyond. Trends Microbiol 2015;23(8):468‐478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Fan Y, Zhao K, Shi Z‐L, Zhou P. Bat coronaviruses in China. Viruses. 2019;11(3):210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wang M, Wu Q, Xu W, et al. Clinical diagnosis of 8274 samples with 2019‐novel coronavirus in Wuhan. medRxiv. 2020. [Google Scholar]
- 84. Malik YS, Sircar S, Bhat S, et al. Emerging novel coronavirus (2019‐nCoV)‐current scenario, evolutionary perspective based on genome analysis and recent developments. Vet Q. 2020;40(1):68‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Rodriguez‐Morales AJ, Bonilla‐Aldana DK, Balbin‐Ramon GJ, et al. History is repeating itself: probable zoonotic spillover as the cause of the 2019 novel Coronavirus Epidemic. Infezioni Med Le. 2020;28(1):3‐5. [PubMed] [Google Scholar]
- 86. Schlottau K, Rissmann M, Graaf A, et al. SARS‐CoV‐2 in fruit bats, ferrets, pigs, and chickens: an experimental transmission study. Lancet Microbe. 2020;1(5):e218‐e225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. AVMA . SARS‐CoV‐2 in animals American Veterinary Medical Association. 2020. https://www.avma.org/resources-tools/animal-health-and-welfare/covid-19/sars-cov-2-animals-including-pets. [Google Scholar]
- 88. Mallapaty S. China set to clamp down permanently on wildlife trade in wake of coronavirus. Nature. 2020. [DOI] [PubMed] [Google Scholar]
- 89. Mallapaty S. Coronavirus can infect cats ‐ dogs, not so much. Nature. 2020. [DOI] [PubMed] [Google Scholar]
- 90. Agriculture USDo . USDA Statement on the Confirmation of COVID‐19 in a Tiger in New York. 2020. https://www.aphis.usda.gov/aphis/newsroom/news/sa_by_date/sa-2020/ny-zoo-covid-19. [Google Scholar]
- 91. Gretebeck LM, Subbarao K. Animal models for SARS and MERS coronaviruses. Curr Opin Virol. 2015;13:123‐129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Sutton TC, Subbarao K. Development of animal models against emerging coronaviruses: from SARS to MERS coronavirus. Virology. 2015;479‐480:247‐258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kim Yl, Kim SG, Kim SM, et al. Infection and rapid transmission of SARS‐CoV‐2 in ferrets. Cell Host Microbe. 2020;27(5):704–709.e2. 10.1016/j.chom.2020.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Weingartl H, Copps J, Drebot M, et al. Susceptibility of pigs and chickens to SARS coronavirus. Emerg Infect Dis. 2004;10(2):179‐184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Wu C, Zheng M, Yang Y, et al. In silico analysis of intermediate hosts and susceptible animals of SARS‐CoV‐2. ChemRxiv. 2020. [Google Scholar]
- 96. Tiwari R, Dhama K, Sharun K, et al. COVID‐19: animals, veterinary and zoonotic links. Vet Q. 2020;40(1):169–182. 10.1080/01652176.2020.1766725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Bao L, Gao H, Deng W, et al. Transmission of SARS‐CoV‐2 via close contact and respiratory droplets among hACE2 mice. J Dis Infect. 2020;222(4):551–555. 10.1093/infdis/jiaa281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Hassan AO, Case JB, Winkler ES, et al. A SARS‐CoV‐2 infection model in mice demonstrates protection by neutralizing antibodies. Cell. 2020;182(3):744–753.e4. 10.1016/j.cell.2020.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Israelow B, Song E, Mao T, et al. Mouse model of SARS‐CoV‐2 reveals inflammatory role of type I interferon signaling. bioRxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Oh DY, Hurt AC. Using the ferret as an animal model for investigating influenza antiviral effectiveness. Front Microbiol. 2016;7:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Roberts A, Lamirande EW, Vogel L, et al. Animal models and vaccines for SARS‐CoV infection. Virus Res. 2008;133(1):20‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Cross RW, Speranza E, Borisevich V, et al. Comparative transcriptomics in Ebola Makona‐infected ferrets, nonhuman primates, and humans. J Infect Dis. 2018;218(suppl_5):S486‐S95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Colman RJ. Non‐human primates as a model for aging. Biochim Biophys Acta (BBA) ‐ Mol Basis Dis. 2018;1864(9 Pt A):2733‐2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Rockx B, Kuiken T, Herfst S, et al. Comparative pathogenesis of COVID‐19, MERS, and SARS in a nonhuman primate model. Science. 2020;368(6494):1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Mizumoto K, Kagaya K, Zarebski A, Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID‐19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill. 2020;25(10):2000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Krammer F. SARS‐CoV‐2 vaccines in development. Nature. 2020;586(7830). 516‐527. [DOI] [PubMed] [Google Scholar]
- 107. Lee J. These 23 companies are working on coronavirus treatments or vaccines — here's where things stand. 2020. https://www.marketwatch.com/story/these-nine-companies-are-working-on-coronavirus-treatments-or-vaccines-heres-where-things-stand-2020-03-06. [Google Scholar]
- 108. DeFrancesco L. COVID‐19 antibodies on trial. Nat Biotechnol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Dinnon KH, Leist SR, Schäfer A, et al. A mouse‐adapted model of SARS‐CoV‐2 to test COVID‐19 countermeasures. Nature. 2020;586(7830):560‐566. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


