Abstract
Background and aims
The outbreak of coronavirus disease 2019 (COVID‐19) caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection has recently spread worldwide and been declared a pandemic. We aim to describe here the various clinical presentations of this disease by examining eleven cases.
Methods
Electronic medical records of 11 patients with COVID‐19 were collected, and demographics, clinical manifestations, outcomes, key laboratory results, and radiological images are discussed.
Results
The clinical course of the eleven cases demonstrated the complexity of the COVID‐19 profile with different clinical presentations. Clinical manifestations range from asymptomatic cases to patients with mild and severe symptoms, with or without pneumonia. Laboratory detection of the viral nucleic acid can yield false‐negative results, and serological testing of virus‐specific IgG and IgM antibodies should be used as an alternative for diagnosis. Patients with common allergic diseases did not develop distinct symptoms and severe courses. Cases with a pre‐existing condition of chronic obstructive pulmonary disease or complicated with a secondary bacterial pneumonia were more severe.
Conclusion
All different clinical characteristics of COVID‐19 should be taken into consideration to identify patients that need to be in strict quarantine for the efficient containment of the pandemic.
Keywords: allergic diseases, case reports, clinical characteristics, coronavirus disease 2019, SARS‐CoV‐2
Clinical manifestations of COVID‐19 range from asymptomatic cases to patients with mild and severe symptoms, with or without pneumonia. Laboratory detection of the viral nucleic acid can yield false‐negative results, and serological testing of virus‐specific IgG and IgM antibodies should be used as an alternative for diagnosis. Patients with common allergic diseases did not develop distinct symptoms and severe courses. Cases with a pre‐existing condition of chronic obstructive pulmonary disease or complicated with a secondary bacterial pneumonia were more severe.

Abbreviations
- COVID-19
coronavirus-19
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- RT-PCR
reverse transcription polymerase chain reaction reverse transcription polymerase chain reaction
- COPD
chronic obstructive pulmonary disease
1. INTRODUCTION
A novel strain of human coronaviruses, the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), named by the International Committee on Taxonomy of Viruses (ICTV),1 has emerged and caused an infectious disease recently referred to as “coronavirus disease 2019” (COVID‐19) by the World Health Organization (WHO). Since the first report of this disease in December 2019 in Wuhan, China,2, 3 COVID‐19 has aggressively spread across the globe. WHO declared it a pandemic on March 11, with over 110 000 patients and rising.4
Clinical studies have indicated that most common manifestations of COVID‐19 are fever, fatigue, and dry cough. Other symptoms include myalgia, chest tightness, dyspnea, nausea, vomiting, and diarrhea. Chest computerized tomography (CT) scans show typical viral pneumonia images with multiple bilateral ground‐glass opacities or consolidation; common laboratory findings are lymphopenia and/or leukopenia.5, 6, 7, 8, 9 According to the practice guidance for diagnosis and treatment of novel coronavirus pneumonia in China (7th trial version) issued by the China National Health Commission, COVID‐19 is diagnosed on the basis of epidemiological history and clinical symptoms as described above, combined with confirmed infection of SARS‐Cov‐2 through one of the following methods: real‐time reverse transcription‐polymerase chain reaction (RT‐PCR) assay, high‐throughput genome sequencing, and serological measurements of antiviral immunoglobulin M (IgM) and G (IgG) antibodies. It is worth noting that it has been previously reported that some individuals infected with SARS‐CoV‐2 are asymptomatic when RT‐PCR tests are confirmed positive,10, 11, 12, 13 and some patients that recovered from COVID‐19 have positive RT‐PCR results again at follow‐up.14 Previous reports indicate that most COVID‐19 patients are middle‐ and old‐aged, often with underlying conditions such as hypertension, diabetes mellitus, and coronary heart diseases (CHD), especially in severe cases.9, 13 However, chronic obstructive pulmonary diseases (COPD) are relatively less common in COVID‐19 patients, with a prevalence of 1.1%‐2.9%.7, 8, 9 In a study involving 140 cases with COVID‐19 on the association between allergies and infection, no patients were found to have asthma or allergic rhinitis.8
As for SARS‐CoV‐2, a novel virus, there are still unmet research needs to further characterize its clinical profile.15 The more we investigate the clinical situations and characteristics of COVID‐19, the more lessons and knowledge we obtain. In this article, eleven confirmed cases are described as examples of the many faces of COVID‐19 clinical manifestations. The data was collected from electronic medical records of three hospitals, Zhongnan Hospital of Wuhan University, Wuhan No.7 Hospital and Wuhan Children’s Hospital. Main clinical data and laboratory results are tabulated in Tables 1 and 2, respectively.
Table 1.
Clinical data of eleven cases
| Type of presentation | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | Case 9 | Case 10 | Case 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Asymptomatic infection | Mild without pneumonia | Negative RT‐PCR but positive Abs | Positive RT‐PCR after recovery | Pneumonia with mild symptoms | Complicated with secondary pneumonia | Pneumonia with allergic rhinitis | Pneumonia with atopic dermatitis | Pneumonia with urticaria | Pneumonia with pre‐existing COPD | Onset with diarrhea | |
| Age | 26 | 22 | 42 | 44 | 46 | 69 | 3 | 2 | 44 | 62 | 43 |
| Gender | Male | Female | Female | Male | Female | Female | Male | Male | Female | Male | Female |
| Pre‐existing condition(s) | None | None | None | None | None | Heart disease | Allergic rhinitis, CAP | Atopic dermatitis, CAP | Chronic urticaria | Hypertension, COPD | None |
| History of smoking | No | No | No | No | No | No | No | No | No | Yes (>20 y) | No |
| Symptoms | None | Sore throat, dry cough | Fever, dry cough, chest tightness, short of breath, loss of appetite, nausea, diarrhea | Cough, dizziness, fatigue | Runny nose, sore throat, cough | Fever, dry cough, headaches, sore throat, myalgia, fatigue | Cough with phlegm | Dry cough, fever | Fever, headache | Fever, dry cough, loss of appetite | Fever, dry cough, diarrhea |
| Chest CT | Normal | Normal | Bilateral pneumonia | Bilateral pneumonia | Bilateral pneumonia | Bilateral pneumonia | Pneumonia in upper left lobe | Bilateral pneumonia | Bilateral pneumonia | Emphysema and bilateral pneumonia | Bilateral pneumonia |
| Treatmentsa | Arbidol, Prezcobix | Arbidol | Moxifloxacin, Arbidol, methylprednisolone | Arbidol, Kaletra | Arbidol, Kaletra, interferon‐α inhalation | Antibiotics, caspofungin, methylprednisolone, invasive mechanical ventilation, ECMO, erythrocytes transfusions | Interferon‐α inhalation | Interferon‐α inhalation | Arbidol | Cefoperazone sulbactam, moxifloxacin, oseltamivir, noninvasive mechanical ventilation | Arbidol, interferon‐α inhalation, methylprednisolone, ambroxol |
| Days from first symptoms to negative RT‐PCR | 2 (from the day when RT‐PCR was positive) | 26 | NA (no positive RT‐PCR) | 14 (positive RT‐PCR again after 14 days from the last discharge) | 11 | > 44 (still positive RT‐PCR when the medical record was obtained) | 26 | 13 | 18 | 34 | 20 |
Abbreviations: Abs, antibodies (against the virus); CAP, community‐acquired pneumonia; COPD, chronic obstructive pulmonary disease; ECMO, extracorporeal membrane oxygenation; NA, not available; RT‐PCR, real‐time reverse transcriptase‐polymerase chain reaction (for detection of viral nucleic acid).
Supportive therapies were included in the treatments for all the cases. Antibiotics were ceftriaxone and moxifloxacin initially and changed to cefoperazone sulbactam, linezolid, and polymyxin later.
Table 2.
Laboratory results of eleven cases
| Type of presentation | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | Case 9 | Case 10 | Case 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Asymptomatic infection | Mild without pneumonia | Negative RT‐PCR but positive Abs | Positive RT‐PCR after recovery | Pneumonia with mild symptoms | Complicated with secondary pneumonia | Pneumonia with allergic rhinitis | Pneumonia with atopic dermatitis | Pneumonia with urticaria | Pneumonia with pre‐existing COPD | Onset with diarrhea | |
| Laboratory results on admission (unit, reference ranges) | |||||||||||
| Leukocytes (×109/L, 3.5 ~ 9.5) | 6.85 | 4.08 | 4.34 | 3.37a | 1.92a | 4.78 | 5.52 | 6.72 | 3.19a | 8.43 | 3.8 |
| Neutrophils (×109/L, 1.8 ~ 6.3) | 4.17 | 2.11 | 3.33 | 1.47a | 1.20a | 3.06 | 2.27 | 3.10 | 1.57a | 7.53b | 2.23 |
| Lymphocytes (×109/L, 1.1 ~ 3.2) | 1.88 | 1.54 | 0.81a | 1.43 | 0.47a | 1.29 | 2.76 | 2.91 | 1.28 | 0.27a | 1.18 |
| Eosinophils (×109/L, 0.02 ~ 0.52) | 0.21 | 0.11 | 0a | 0.17 | 0a | 0a | NA | NA | 0.02 | NA | 0a |
| Basophils (×109/L, 0 ~ 0.06) | 0.03 | 0 | 0 | 0 | 0.01 | 0.01 | NA | NA | 0 | NA | NA |
| Monocytes (×109/L, 0.1 ~ 0.6) | 0.56 | 0.32 | 0.20 | 0.30 | 0.24 | 0.42 | NA | NA | 0.32 | NA | NA |
| Hemoglobin (g/L, 115 ~ 150) | 153.6 | 133 | 122 | 141 | 97.6a | 118 | 125 | 134 | 130 | NA | 78.8a |
| Platelets (×109/L, 125 ~ 350) | 248 | 314 | 195 | 149 | 167 | 127 | 241 | 192 | 164 | NA | NA |
| CRP (mg/L, 0 ~ 3) | 1.5 | 0.4 | 49.9b | 2.4 | 3.4b | 60.41b | < 3.0 | 10.0b | 29.2b | > 160b | 3.67b |
| SAA (mg/L, 0 ~ 10) | 9.2 | NA | NA | NA | 36.84b | NA | NA | 93.22b | NA | NA | 83.7b |
| PCT (ng/ml, 0 ~ 0.1) | < 0.05 | 0.031 | 0.084 | 0.057 | < 0.05 | 0.05 | 0.08 | 0.08 | 0.045 | 0.39b | NA |
| Sputum culture | NA | NA | NA | NA | NA | Gram‐positive cocci and gram‐negative bacilli | NA | NA | NA | NA | NA |
Abbreviations: Abs, antibodies (against the virus); COPD, chronic obstructive pulmonary disease; NA, not available; RT‐PCR, real‐time reverse transcriptase‐polymerase chain reaction (for detection of viral nucleic acid).
Denotes a decreased result.
Denotes an increased result.
1.1. Case 1: Asymptomatic SARS‐CoV‐2 infection
A 26‐year‐old male nurse was screened for COVID‐19 on February 14, 2020, after contact with an infected person. RT‐PCR assay with an oropharyngeal swab sample tested positive for SARS‐CoV‐2. Chest CT images showed no signs of pneumonia. The patient was admitted to the isolated ward and treated with antiviral drugs including Arbidol and Prezcobix (darunavir and cobicistat tablets). All laboratory results were within normal ranges. The patient was discharged 4 days later after testing negative in two consecutive RT‐PCR assays. The patient did not experience any symptoms before and during hospitalization.
1.2. Case 2: A mild symptomatic case with negative chest CT results
On January 19, a 22‐year‐old female medical staff began to have a sore throat with dry cough after a night shift, without developing rhinitis. Symptoms were relieved by oral common cold medications including Chinese traditional medicine (CTM). She felt discomfort with myalgia on January 25 and tested positive for SARS‐CoV‐2, as detected in a RT‐PCR assay with an oropharyngeal swab specimen. The complete blood count (CBC) was within normal ranges, and chest CT scan was negative for any signs of pneumonia. Two additional RT‐PCR results (January 31 and February 7) were positive for novel coronavirus nuclear acid and with normal chest CT images on both days. She was admitted to the designated hospital on February 9. During hospitalization, all the CBC and biochemical measurements were in normal ranges, and the chest CT scan on February 15 remained normal. She experienced only mild symptoms such as a sore throat and fatigue, without any signs of pneumonia such as cough and chest tightness. The patient recovered after treatment with antiviral therapy (Arbidol) and supportive care. She was discharged on February 22 after three consecutive negative RT‐PCR results. We suggest that this case represents a mild COVID‐19 without pneumonia.
1.3. Case 3: Typical COVID‐19 symptoms and radiological changes in a patient with negative RT‐PCR results for SARS‐CoV‐2 and positive IgG and IgM antibodies against the virus
After contacting with feverish patients, a 42‐year‐old woman developed fever, with a maximum temperature of 38.7°C, accompanied by a dry cough and sore throat on January 23. An immediate chest CT scan at the designated clinic showed normal images. She was self‐isolated at home and took some common cold medications, CTM and antipyretics to relieve symptoms. The fever ameliorated but the cough persisted. A week later, a second chest CT scan revealed pneumonia in the left lung (Figure 1A). An oropharyngeal swab specimen was collected and tested negative RT‐PCR for the novel coronavirus nucleic acid. Despite treatment with antipyretics, her fever reappeared (39.2°C). She developed chest tightness, shortness of breath, loss of appetite, nausea, and diarrhea. On February 6, the third chest CT scan showed bilateral multiple ground‐glass opacities indicating viral pneumonia (Figure 1B) and she was admitted to the designated hospital immediately. On admission, rales were noted in both lungs on auscultation, lymphocyte count slightly decreased [0.81 × 109/L, reference range: (1.1 ~ 3.2) ×109/L], and the concentration of serum high‐sensitivity C‐reactive protein (hsCRP) increased [49.9 mg/L, reference range: (0 ~ 3) mg/L], with leukocyte count and procalcitonin (PCT) concentration within normal ranges. She was treated with antibiotics (moxifloxacin), antiviral drug (Arbidol), methylprednisolone on gradually decreased dosage for six days, and supportive care. The four RT‐PCR assays on throat swab specimens for SARS‐CoV‐2 (February 7, 11, 17 and 25) and other common respiratory pathogens tested negative during hospitalization. On February 29, serological antibodies tests against SARS‐CoV‐2 were conducted and found positive IgM and positive IgG antibodies, confirming the diagnosis of COVID‐19. With significant reduction in clinical symptoms and CT manifestations, the patient was discharged on March 1. We suggest that this case represents a COVID‐19 pneumonia without detectable virus in oropharyngeal specimens.
Figure 1.
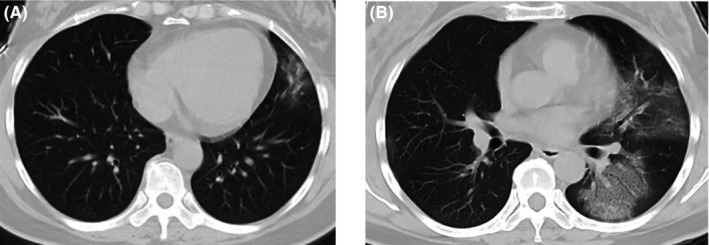
Chest CT images of case 3. (A) Patchy ground‐glass opacities in left upper lobe on January 30; (B) progressed bilateral multiple ground‐glass opacities on February 6
1.4. Case 4: Positive RT‐PCR results 2 weeks after recovery
A 44‐year‐old man was admitted to the designated hospital as COVID‐19 infection was confirmed on February 2. Symptoms included cough, dizziness, and fatigue for one week, with bilateral ground‐glass opacities on chest CT images. RT‐PCR on the throat swab sample was positive for SARS‐CoV‐2. Most laboratory tests were within normal ranges, except for mild leukopenia [3.37 × 109/L, reference range: (3.5 ~ 9.5) × 109/L], and neutropenia [1.47 × 109/L, reference range: (1.8 ~ 6.3) ×109/L]. Three consecutive RT‐PCR assays on oropharyngeal swab specimens were conducted during hospitalization. The results tested positive on February 6 and negative on February 9 and 12. The patient received antiviral drugs including Arbidol and Kaletra (lopinavir and ritonavir tablets) and was discharged on February 13 when chest CT images showed that the bilateral lesions were mostly absorbed. Even though he was asymptomatic, he was readmitted to the hospital two weeks later as he tested positive in a RT‐PCR assay for SARS‐CoV‐2 using a sample collected from an oropharyngeal swab. During the second hospitalization, the CBC and all biochemical tests were within normal ranges. He remained in the hospital as SARS‐CoV‐2 detection by a RT‐PCR assay was positive on March 3 and 7. We suggest that this case is representative of a long‐term virus carrier.
1.5. Case 5: Typical pneumonia signs on chest CT but with only mild respiratory symptoms and normal CRP levels
A 46‐year‐old woman with cold‐like symptoms including runny nose and sore throat came to the designated clinic. A chest CT scan three days later (January 23) showed multiple ground‐glass opacities in bilateral lower lungs, indicating possible viral pneumonia (Figure 2A, B and C). The cold‐like symptoms rapidly improved, whereas cough with little white sputum appeared the next day. The RT‐PCR assay of a throat swab specimen tested positive for SARS‐CoV‐2 on January 27, and the patient was immediately hospitalized. On admission, physical examinations and pulmonary auscultation were normal; laboratory tests indicated a reduction in leukocytes (1.92 × 109/L), neutrophils (1.20 × 109/L), lymphocytes (0.47 × 109/L), and eosinophils (0 × 109/L) counts, normal CRP, and PCT concentrations, and slightly increased concentration of serum amyloid A [SAA, 36.84 mg/L, reference range: (0 ~ 10) mg/L]. The second chest CT scan on January 31 showed progression of bilateral multiple ground‐glass opacities with scattered consolidation, compared to the first CT scan (Figure 2D, E and F). Fatigue, cough, fever, and diarrhea symptoms gradually improved after treatment with antiviral medication (Arbidol, Kaletra and inhalation of interferon‐α) and supportive care. The patient was discharged on February 4 after two consecutive negative RT‐PCR results of the coronavirus nucleic acid. We consider this case as a moderate COVID‐19 pneumonia.
Figure 2.
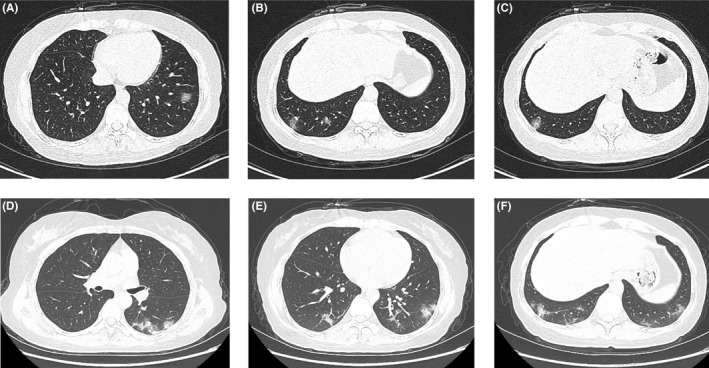
Chest CT images of case 5. (A‐C) Multiple focal ground‐glass opacities in both lower lobes on January 23; (D‐E) bilateral multiple patchy ground‐glass opacities mixed consolidation and fibrous stripes on January 31
1.6. Case 6: A severe COVID‐19 patient complicated by secondary bacterial pneumonia
A 69‐year‐old woman had persistent fever (>38°C) accompanied by a dry cough, headache, sore throat, myalgia, and fatigue for three days. A chest CT scan one day prior to admission revealed bilateral scattered patchy opacities (Figure 3A). The patient had an uncertain history of heart disease. She was admitted into the designated hospital on January 20. On admission, she had normal vital signs except for low‐grade fever (37.5°C). Bilateral rales were detected on auscultation. The CBC was normal except for the development of eosinopenia (0 × 109/L) and an elevated erythrocyte sedimentation rate (ESR) and CRP concentration (39 mm/h and 60.41 mg/L, respectively). PCT, liver and myocardial enzymes, brain natriuretic peptide (BNP), and renal function were all within normal ranges. No other respiratory pathogens were detected by serological antibodies testing and nucleic acid testing. However, gram‐positive cocci and gram‐negative bacilli were detected in sputum culture. Broad‐spectrum antibiotics were administrated intravenously, together with expectorants and supplemental oxygen delivered by nasal cannula. On January 23, the second chest CT scan revealed bilateral extended ground‐glass opacities with scattered consolidation, indicating rapid progression of the disease (Figure 3B). The patient was transferred to the intensive care unit (ICU) immediately the next day. Remarkable abnormalities in the CBC were detected on January 29, including increased neutrophils (10.67 × 109/L), leukocytes (11.73 × 109/L), and decreased lymphocytes (0.51 × 109/L), and on February 4, decreased erythrocytes (2.40 × 1012/L) and platelets (73 × 109/L). In addition, there was a significant increase in inflammatory indicators, such as PCT, CRP, and D‐dimer. On February 3, the patient suffered respiratory and cardiac failure and was subjected to invasive mechanical ventilation and extracorporeal membrane oxygenation (ECMO). Unfortunately, during the operation of ECMO she suffered a sudden cardiac arrest and survived by rescue of cardiopulmonary resuscitation (CPR). On February 10, bedside bronchoscopy and bronchial lavage were performed, revealing hyperemia in mucous membranes of the trachea and bronchi with bleeding tendency and a large amount of yellow sticky sputum obstructing the entrance of the bilateral main bronchi and some bronchial lumens, which were aspirated completely. The bedside chest X‐ray showed progression of the pneumonia, since the bilateral diffuse opacities, so‐called “white lungs,” with air bronchogram occurred on February 15 (Figure 3C, D and E). Gram‐negative bacilli were found in the second sputum culture. Six RT‐PCR assays for SARS‐CoV‐2 remained positive during hospitalization. The patient was treated with potent broad‐spectrum antibiotics combined with antifungal agents, methylprednisolone, repeated erythrocytes transfusions, and supportive care. This severe patient was still hospitalized in the ICU when we obtained her electronic medical record on March 1. We regard this patient as a case suffering from a severe secondary bacterial pneumonia concomitant with COVID‐19 infection.
Figure 3.
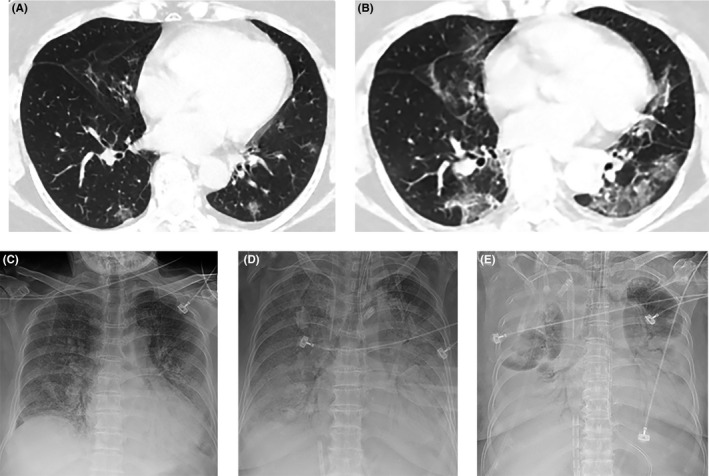
Chest CT and X‐ray images of case 6. (A) Bilateral scattered patchy opacities on January 19; (B) bilateral extended ground‐glass opacities with scattered consolidation on January 23; (C‐E) bilateral progressive consolidation and pleural effusion on February 2, 8, and 15, respectively
1.7. Case 7: A pediatric COVID‐19 patient with a history of allergic rhinitis
A 3‐year‐old boy suffered from intermittent cough with phlegm for more than ten days. He was diagnosed with allergic rhinitis according to the symptoms of sneezing, itchy, and running nose in Wuhan Children's Hospital one year ago. In addition, the boy also suffered from community‐acquired pneumonia (CAP) during the past two years (details unavailable). Treatment with azithromycin medication was ineffective in improving the symptoms. Although CBC and CRP laboratory tests (performed twice) were within normal ranges and the screening of common respiratory pathogens was negative, the chest CT scan showed signs of pneumonia in the left upper lobe (Figure 4A). He was admitted to the hospital on January 28; no abnormal signs were noted during physical examination. Further CBC and biochemical parameters analyses were within normal ranges. Serum immunoglobulin E (IgE) level was normal (26 IU/mL). RT‐PCR assays (in triplicate) on throat swab specimens for SARS‐CoV‐2 were positive during hospitalization. Symptoms were relieved after inhalation of interferon‐α and supportive care. The patient was discharged on February 17 after testing negative on two consecutive RT‐PCR results. This case study represents a pediatric patient with COVID‐19 pneumonia with a history of allergic rhinitis.
Figure 4.
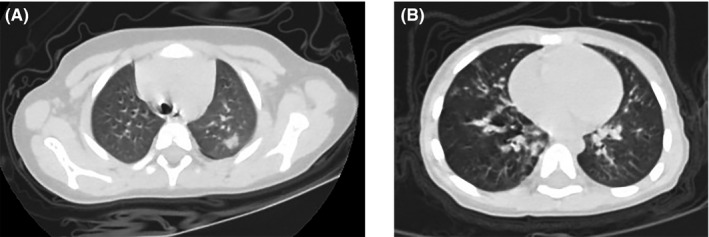
Chest CT images of two children with COVID‐19. (A) On January 25, case 7 showed isolated patchy consolidation in the upper left lobe; (B) On January 28, case 8 showed bilateral diffuse patchy opacities distributed around the bronchovascular area
1.8. Case 8: A pediatric COVID‐19 patient with a history of atopic dermatitis
After contact with children with a cough, a 28‐month‐old boy began to cough and developed a strong fever (39.2°C) two days later. He was admitted to the hospital a week later on January 28. According to information provided by his parents, he was previously diagnosed with atopic dermatitis and suffered from repetitive respiratory infections during winter. On admission, physical examination revealed a body temperature of 37.8°C, respiratory rate of 28 breaths per minute, pulse of 120 beats per minute, and rales in both lungs on auscultation, without additional signs. Chest CT images showed bilateral pneumonia (Figure 4B). All the laboratory results were within normal ranges, including CBC and biochemical parameters, except for elevated levels of serum IgE (173 IU/mL). RT‐PCR results were positive for SARS‐CoV‐2. No other common respiratory pathogens were identified except for positive cytomegalovirus‐IgM. The patient was treated with interferon‐α inhalation and supportive care. With the relief of symptoms and two consecutive negative RT‐PCR results, he was discharged on February 6. This patient was a pediatric case with COVID‐19 pneumonia with a background of atopic dermatitis.
1.9. Case 9: A COVID‐19 patient with a history of urticaria
A 44‐year‐old woman had fever and headaches, with an onset of symptoms from January 18. Chest CT images revealed bilateral ground‐glass opacities and a RT‐PCR assay was positive for SARS‐CoV‐2. She was diagnosed as laboratory‐confirmed COVID‐19 and admitted to the designated hospital on January 23. The patient had over a two‐year history of chronic spontaneous urticaria and frequently took oral H1‐antihistamines. On admission, physical examination revealed no abnormal signs. Laboratory tests showed a slight decrease of leukocytes (3.19 × 109/L) and neutrophils (1.57 × 109/L) and a mildly elevated CRP concentration (29.2 mg/L), with all other tested parameters within normal ranges. Common respiratory pathogens were not detected. Symptoms improved after antiviral treatment (Arbidol) and supportive care. All of the RT‐PCR assays performed during hospitalization for the coronavirus nucleic acid were negative. She was discharged on February 14. This case exemplifies an adult with COVID‐19 pneumonia with a background of chronic spontaneous urticaria.
1.10. Case 10: A COPD patient with COVID‐19 and influenza A pneumonia
A 62‐year‐old man had a persistent fever with temperature > 38.5°C, accompanied by dry cough and loss of appetite for 20 days. He was admitted to the hospital on January 22. Chest CT images showed signs of emphysema and pneumonia (Figure 5). He was a smoker for more than twenty years and had been diagnosed with hypertension and COPD (details unavailable). On admission, physical examination indicated a heart rate of 101 beats per minutes, blood pressure of 112/58 mmHg, respiratory rate of 21 breaths per minutes, oxygen saturation of 92% while breathing ambient air, and rough respiratory sounds on auscultation, without other abnormal signs. A CBC showed slightly increased neutrophils (7.53 × 109/L) and markedly decreased lymphocytes (0.27 × 109/L). Other abnormal results of laboratory parameters included significantly increased CRP concentration (>160 mg/L) and slightly increased PCT concentration (0.39 ng/mL). Influenza A virus IgM was positive, whereas other common respiratory pathogens were not found. The RT‐PCR assay for SARS‐CoV‐2 nucleic acid was positive on January 31 after a previous negative result. Using oxygen supplementation and noninvasive mechanical ventilation, the oxygen saturation could be kept above 95%. Other therapies included antibiotics (cefoperazone sulbactam and moxifloxacin) for targeting the underlying bacterial infection, antiviral drugs (oseltamivir) and supportive care. His symptoms gradually improved, with absorption of inflammatory exudation on CT images and laboratory indicators back to normal levels. After two consecutive negative RT‐PCR results, the patient was discharged on February 9.
Figure 5.
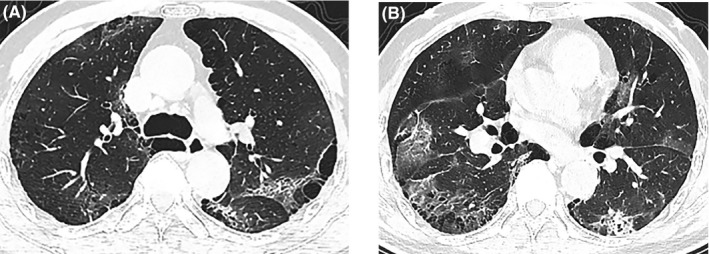
Chest CT images of case 10. (A, B) Images of two layers showing emphysema and bullae in both lungs and bilateral ground‐glass opacities and consolidation on January 22
1.11. Case 11: A COVID‐19 patient presented with diarrhea in addition to respiratory symptoms
A 43‐year‐old woman developed fever and a dry cough for two days, accompanied by nonbloody diarrhea (5 to 10 times per day) without obvious abdominal cramps. The RT‐PCR result on a throat swab specimen for viral nucleic acid was weakly positive. She was admitted to the hospital on January 31 and underwent physical examination without abnormal signs. The CBC analysis was normal, except for the development of eosinopenia (0 × 109/L). Other laboratory tests showed slightly increased concentration of CRP and SAA (3.67 mg/L and 83.7 mg/L, respectively), with all the rest of biochemical parameters within normal ranges. Chest CT images presented bilateral multiple ground‐glass opacities on February 2 (Figure 6A). Two RT‐PCR assays for SARS‐CoV‐2 nucleic acid tested positive during hospitalization. The patient received various treatments including antiviral drugs (Arbidol and interferon‐α inhalation), expectorant, methylprednisolone on gradually decreased dosages for five days, and supportive care. The second chest CT scan after a week showed partial absorption of lesions (Figure 6B). The patient's symptoms, including diarrhea, disappeared a few days after treatment. After two consecutive negative results of viral nucleic acid tests, she was discharged on February 20. This case is of high interest as the patient experienced diarrhea in the initial symptoms.
Figure 6.
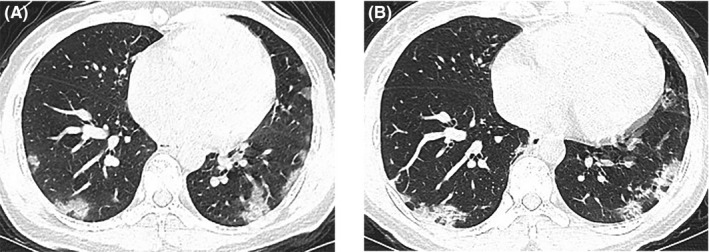
Chest CT images of case 11. (A) Bilateral multiple ground‐glass opacities and consolidation on February 2; (B) partly absorbed bilateral ground‐glass opacities and consolidation on February 9
2. DISCUSSION
Herein, we present eleven different clinical manifestations of COVID‐19. Apart from the asymptomatic case in our report, several previous case reports have also described the absence of any symptoms in patients with laboratory‐confirmed COVID‐19, and they even retain the ability of disease transmission.10, 16 It is of particular interest to investigate if the asymptomatic cases are caused by the immune system, viral load, or a different attenuated viral strain.17 In these cases, the virus has been detected in the pharyngeal specimens, and it is very unlikely to have a different strain in this epidemic condition. It is possible that the virus did not sufficiently stimulate the innate immune response to develop symptoms of fever and fatigue. In addition, there were no respiratory symptoms, such as cough, suggesting a low‐level mucosal irritation. Asymptomatic but infective status has been reported in many viruses, such as respiratory, hepatitis, and diarrhea viruses, as well as in other major human viruses, such as cytomegalovirus or herpes viruses.18, 19, 20 In a recent COVID‐19 study, the viral load in upper respiratory specimens of asymptomatic patients was found in similar levels to that of symptomatic patients, suggesting a similar transmission potential of asymptomatic or minimally symptomatic patients.21 In a large‐scale epidemiological survey of all COVID‐19 cases through February 11, 2020, reported by the Chinese Center for Disease Control and Prevention, including 72,314 laboratory‐confirmed cases, 889 (1.2%) asymptomatic cases were noted.13 However, it should be noted that a major fraction of tests were performed in symptomatic individuals. A better epidemiologic picture of asymptomatic carriers is expected to be determined after performing a large serological study for the detection of virus specific‐IgG antibodies in an exposed population without any age and comorbidity bias. For now, it is important to consider that some of the individuals infected with SARS‐CoV‐2 may not display any symptoms and may become a potential source of infection. Therefore, quarantine measures should also be enforced for those individuals that are asymptomatic and have a history of epidemiological contact.
It has been previously reported that some patients infected with SARS‐CoV‐2 can develop coexisting gastrointestinal symptoms at the onset of symptoms, such as in case 11 where the patient experienced diarrhea, in addition to the typical fever and cough. Previous large‐scale clinical investigations indicated the common gastrointestinal symptoms of COVID‐19 patients were nausea/vomiting and diarrhea, with a prevalence of 5.0% and 3.8%, respectively.9 Not only can the virus be detected in fecal specimens,22 but also the viral nucleic acid testing on rectal swabs remained positive even after nasopharyngeal testing had become negative.23 These findings demonstrate that the novel coronavirus can exist and induce infections in the digestive system and that the viral shedding from the intestinal tract may be greater and last longer than that from the respiratory tract. Therefore, gastrointestinal symptoms and possible fecal‐oral transmission of COVID‐19 should be taken seriously and warrant further investigation.
The RT‐PCR assay for viral nucleic acid is the current “gold standard” method to detect SARS‐CoV‐2 and diagnose confirmed cases. However, this testing can yield false‐negative results and is limited by its poor sensitivity, due to possible testing errors or insufficient collection of samples. In our report, case 3 had persistent negative RT‐PCR assay results and was later identified through the detection of IgM and IgG antibodies against the novel coronavirus. Detection of SARS‐CoV‐2‐specific IgM represents an essential tool to confirm cases that are negative for viral nucleic acid. It is now important to focus on the reasons of obtaining false‐negative results and confirming the quality and specificity of many serodiagnosis kits commercially available.
Case 4 is a patient whose negative RT‐PCR result of SARS‐CoV‐2 turned positive after two weeks, even though he had three consecutive negative results prior to discharge of the first hospitalization. A previous report by Lan et al14 also reported four Chinese patients with COVID‐19, who met the criteria for hospital discharge or discontinuation of quarantine, developed positive RT‐PCR test results 5 to 13 days later. The following four discharge criteria were issued by the China National Health Commission: (a) no fever for at least 3 days; (b) significant improvement in respiratory symptoms; (c) substantial improvement in chest CT images; and (d) two consecutive negative RT‐PCR results of SARS‐CoV‐19 nucleic acid (at least with a 24‐hour interval). Despite meeting all of the above criteria, some patients’ RT‐PCR results test positive again sometime after discharge. This may be caused by incomplete viral clearance, inappropriate immune response development, incorrect sampling, false‐negative, and/or false‐positive RT‐PCR results. Reinfection is unlikely under the strict quarantine measures which require additional 14‐day isolation and medical observation after discharge. It is necessary to isolate patients with recurrent positive RT‐PCR results and continue treatment until they meet the discharge criteria again. In a recent study, 25 patients with reoccurrence of RT‐PCR results were reported, and the median time from their last negative result to turning positive was 6 days.24 This is the reason why strict quarantine and follow‐up of COVID‐19 patients is of upmost importance.
Some studies suggest that changes in typical chest CT images can be used as an early diagnostic tool for COVID‐19.25, 26 To reduce the undiagnosed rate of the disease, in the 5th trial version of practice guidance for the diagnosis and treatment of novel coronavirus pneumonia in China (February 4), suspected cases with typical radiographic features of viral pneumonia were defined as “clinically diagnosed cases of COVID‐19,” regardless of the result of RT‐PCR assay. Recently, a diagnosis kit of IgM and IgG antibodies against SARS‐CoV‐2 has been developed and applied in clinical practice, with a high detection sensitivity and specificity of 88.66% and 90.63%, respectively.27 Current practice guidance for the diagnosis and treatment of novel coronavirus pneumonia in China (7th trial version, March 3) defined a positive serum IgM and IgG detection result as an alternative method for confirming infection.
We presented two pediatric COVID‐19 patients, with either a history of allergic rhinitis or atopic dermatitis, which are common allergic diseases in children. As we reported in a recent study, allergy does not appear to be a predisposing factor for COVID‐19.8 The two children presented as ordinary types of COVID‐19 and were both treated with interferon‐α inhalation as a nonspecific antiviral therapy, which was one of the trial methods recommended in the practice guidance of novel coronavirus pneumonia in China. The adult patient with chronic urticaria in case 9 was also classified as an ordinary type as well. These and previous results indicate that allergic diseases may not be an aggravating factor of the disease. Previous studies have demonstrated that respiratory virus infections represent risk factors for asthma exacerbation and even have prolonged impact on the development of asthma.28, 29 Given the association between virus infection and asthma,30 it is worth carefully monitoring asthmatic patients in this coronavirus epidemic. However, in pediatric cases, we did not find COVID‐19 patients with a history of asthma (unpublished data). Maybe a distinct type 2 immune response may contribute to this low prevalence of asthma and allergy patients in COVID‐19. The interaction between SARS‐CoV‐2 and asthma remains to be further investigated, especially considering that current medical resources have been mostly focused on COVID‐19.
Studies have demonstrated that COVID‐19 can present various manifestations and severities, and older age and multiple comorbidities including hypertension, diabetes, and CHD are risk factors for the development and severity of infection.9, 31 Even though signs of pneumonia appear on chest CT images, the patient may only experience mild symptoms similar to an upper respiratory infection, as in case 5. If the patient had a decreased lung function caused by a chronic respiratory disease, such as COPD (case 10), or developed complications such as a secondary lung infection (case 6), they were more likely to develop more severe symptoms and need advanced supportive measures including mechanical ventilation, ECMO, and so on. Early diagnosis of secondary infections, such as overlapping bacterial pneumonia, is essential for improved patient care. Bacterial and fungal surveillance of the sputum is necessary if neutrophilic leukocytosis appears with worsening of chest radiology or if CRP suddenly increases in parallel to deterioration of a hospitalized case. A broad‐spectrum antibiotic should be added to treatment as patient deterioration due to secondary infection is relatively fast.
In summary, the existence and potential transmissibility of asymptomatic cases make the careful monitoring and quarantine of COVID‐19 infected patients more important and complicated. The clinical cases presented herein demonstrate the various clinical manifestations of COVID‐19; symptoms may be absent, mild, or severe. Since the RT‐PCR result for SARS‐CoV‐2 nucleic acid can be false‐negative, to some extent, individuals who have a history of epidemiological contact or develop typical symptoms/signs should be seriously considered as a COVID‐19 case in epidemic situations. Serological detection of IgM and IgG antibodies against the coronavirus can be an effective alternative or complementary testing method. Patients with high‐risk factors for COVID‐19 should be monitored carefully and subjected to a full diagnostic procedure, with comprehensive treatment.
AUTHOR CONTRIBUTIONS
Xiang Dong and Yadong Gao collected, organized the clinical data, and prepared the manuscript. Yiyuan Cao and Xiaoxia Lu contributed to the collection of some cases, and Yiyuan Cao interpreted the radiological images. Jinjin Zhang was involved in the preparation of the manuscript. Hui Du and Youqin Yan were involved in the clinical work. Yadong Gao and Cezmi A. Akdis designed the study and reviewed the manuscript.
ACKNOWLEDGMENTS
The authors thank Anna Globinska for preparation of the Graphical Abstract. We would like to express great gratitude and respect to all the healthcare professionals and others who have dedicated themselves to combatting COVID‐19.
Dong X, Cao Y‐Y, Lu X‐X, et al. Eleven faces of coronavirus disease 2019. Allergy. 2020;75:1699–1709. 10.1111/all.14289
Dong, Cao and Lu contributed equally to this work.
Contributor Information
Cezmi A. Akdis, Email: akdisac@siaf.uzh.ch.
Ya‐dong Gao, Email: gaoyadong@whu.edu.cn.
REFERENCES
- 1. Coronaviridae Study Group of the International Committee on Taxonomy of Viruses . The species Severe acute respiratory syndrome‐related coronavirus: classifying 2019‐nCoV and naming it SARS‐CoV‐2. Nat Microbiol. 2020;5:536–544. 10.1038/s41564-020-0695-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhu N, Zhang D, Wang W, et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382:727‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li Q, Guan X, Early WUP, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med. 2020;382:1199‐1207. 10.1056/NEJMoa2001316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization (WHO) . Coronavirus disease (COVID‐2019) situation reports. https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019/situation‐reports. Accessed March 11, 2020.
- 5. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang JJ, Dong X, Cao YY, et al. Clinical characteristics of 140 patients infected with SARS‐CoV‐2 in Wuhan, China. Allergy. 2020;75:1730‐1741. 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 9. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chan JF, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person‐to‐person transmission: a study of a family cluster. Lancet. 2020;395:514‐523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lin C, Ding Y, Xie B, et al. Asymptomatic novel coronavirus pneumonia patient outside Wuhan: The value of CT images in the course of the disease. Clin Imaging. 2020;63:7‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hu Z, Song C, Xu C, et al. Clinical characteristics of 24 asymptomatic infections with COVID‐19 screened among close contacts in Nanjing, China. Sci China Life Sci. 2020. 10.1007/s11427-020-1661-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team . The Epidemiological Characteristics of an Outbreak of 2019 Novel Coronavirus Diseases (COVID‐19) — China, 2020. China CDC Weekly. 2020;2:113‐122. [PMC free article] [PubMed] [Google Scholar]
- 14. Lan L, Xu D, Ye G, et al. Positive RT‐PCR test results in patients recovered from COVID‐19. JAMA. 2020. 10.1001/jama.2020.2783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fauci AS, Lane HC, Redfield RR. Covid‐19 — navigating the uncharted. N Engl J Med. 2020;382:1268‐1269. 10.1056/NEJMe2002387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID‐19. JAMA. 2020. 10.1001/jama.2020.2565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tang X, Wu C, Li X, et al. On the origin and continuing evolution of SARS‐CoV‐2. Nat Sci Rev. 2020. 10.1093/nsr/nwaa036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goswami R, Shair K, Gershburg E. Molecular diversity of IgG responses to Epstein‐Barr virus proteins in asymptomatic Epstein‐Barr virus carriers. J Gen Virol. 2017;98:2343‐2350. [DOI] [PubMed] [Google Scholar]
- 19. Mori K, Konishi N, Suzuki Y, et al. Comparison between patients with norovirus‐related gastroenteritis and asymptomatic carriers with respect to distribution of antibody‐complexed viral particles and intestinal flora. J Med Virol. 2018;90:1882‐1887. [DOI] [PubMed] [Google Scholar]
- 20. Bonadonna L, La Rosa G. A review and update on waterborne viral diseases associated with swimming pools. Int J Environ Res Public Health. 2019;16(2):166. 10.3390/ijerph16020166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zou L, Ruan F, Huang M, et al. SARS‐CoV‐2 Viral Load in Upper Respiratory Specimens of Infected Patients. N Engl J Med. 2020;382(12):1177‐1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang W, Xu Y, Gao R, et al. Detection of SARS‐CoV‐2 in different types of clinical specimens. JAMA. 2020. 10.1001/jama.2020.3786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xu Y, Li X, Zhu B, et al. Characteristics of pediatric SARS‐CoV‐2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020. 10.1038/s41591-020-0817-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yuan J, Kou S, Liang Y, Zeng J, Pan Y, Liu L. Clinical characteristics on 25 discharged patients with COVID‐19 Virus returning. medRxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bernheim A, Mei X, Huang M, et al. Chest CT Findings in Coronavirus Disease‐19 (COVID‐19): Relationship to Duration of Infection. Radiology. 2020;200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huang P, Liu T, Huang L, et al. Use of chest CT in combination with negative RT‐PCR Assay for the 2019 novel coronavirus but high clinical suspicion. Radiology. 2020;295(1):22‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li Z, Yi Y, Luo X, et al. Development and clinical application of A rapid IgM‐IgG combined antibody test for SARS‐CoV‐2 infection diagnosis. J Med Virol. 2020. 10.1002/jmv.25727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Papadopoulos NG, Christodoulou I, Rohde G, et al. Viruses and bacteria in acute asthma exacerbations–a GA2LEN‐DARE systematic review. Allergy. 2011;66:458‐468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Leino A, Lukkarinen M, Turunen R, et al. Pulmonary function and bronchial reactivity 4 years after the first virus‐induced wheezing. Allergy. 2019;74:518‐526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gilles S, Blume C, Wimmer M, et al. Pollen exposure weakens innate defense against respiratory viruses. Allergy. 2020;75(3):576‐587 [DOI] [PubMed] [Google Scholar]
- 31. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054‐1062. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]


