Abstract
There is evidence that the current outbreak of the novel coronavirus SARS-CoV-2, which causes COVID-19, is of animal origin. As with a number of zoonotic pathogens, there is a risk of spillover into novel hosts. Here, we propose a hypothesized conceptual model that illustrates the mechanism whereby the SARS-CoV-2 could spillover from infected humans to naive wildlife hosts in North America. This proposed model is premised on transmission of SARS-CoV-2 from human feces through municipal waste water treatment plants into the natural aquatic environment where potential wildlife hosts become infected. We use the existing literature on human coronaviruses, including SARS CoV, to support the potential pathways and mechanisms in the conceptual model. Although we focus on North America, our conceptual model could apply to other parts of the globe as well.
Keywords: COVID-19, Bats, Raccoons, Waste water treatment plants, Sewage, Spillback
Graphical abstract
Highlights
- •
The pandemic of the novel coronavirus SARS-CoV-2 likely originated from bats or other wildlife species.
- •
We propose a conceptual model on SARS-CoV-2 spillover from infected humans to naive wildlife hosts in North America.
- •
We hypothesize that SARS-CoV-2 is transmitted via sewage into the natural aquatic environment where wildlife become infected.
- •
Wildlife surveillance near waste water treatment plants would elucidate whether SARS-CoV-2 has spilled over into wildlife.
1. Introduction
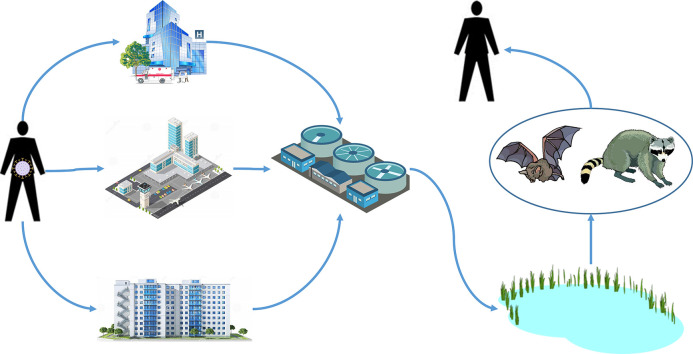
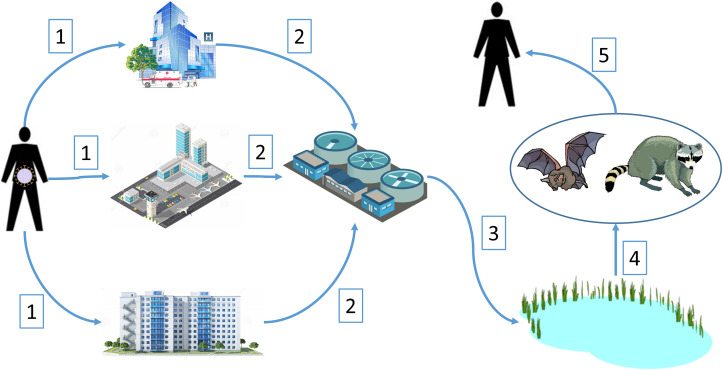
As with SARS and MERS, the recent outbreak of a novel coronavirus (COVID-19) is suspected to have a wildlife origin, with likely ultimate origins in a bat species and possibly with more proximal origins from an intermediate animal host (Ji et al., 2020). With the global spread of the virus among humans, we are concerned that this novel virus has the potential to spillover from infected humans to native wildlife that could subsequently serve as new reservoir hosts for the virus. In this way, the virus may become entrenched in areas outside of its region of origin and available for future outbreaks. However, for this to occur there are a number of hurdles that the virus would need to overcome to infect a novel wildlife host. Here, we propose a plausible mechanism where SARS-CoV-2, the pathogen causing the disease COVID-19, could spillover from infected humans into novel wildlife hosts in North America. We developed a conceptual model for this hypothesized mechanism (Fig. 1 ), which we outlined as five premises supported from the existing literature on shedding of coronaviruses by humans, survivability of these viruses, and documented pathways for movement of coronaviruses into the natural environment. Although we focused on North America, our conceptual model could also be applied to other parts of the globe.
Fig. 1.
Conceptual model of spillover of novel coronavirus SARS-CoV-2 into new wildlife hosts in North America and subsequent spillback into the human population. Numbered pathways are described in the text.
2. Infected people shed coronavirus in feces
For our conceptual model to be plausible, we assume that coronaviruses can be shed in the feces of infected humans. This assumption has been supported by research on other coronaviruses (Yeo et al., 2020). For example, MERS coronavirus was detected in 14.6% and 2.4% of fecal and urine samples, respectively, from 37 infected individuals (Corman et al., 2015), while SARS CoV was shed in the feces of 56 infected individuals for a median of 27 days, with 4 individuals shedding for over 100 days (Liu et al., 2004). In addition, 38.4% of SARS-infected patients experienced diarrhea, SARS-CoV RNA was detected in 16–73% of the stool samples, and cultured from the small intestines of 5 patients (Leung et al., 2003; Yeo et al., 2020). SARS-CoV also remained infectious in diarrheal fecal samples at room temperature for up to 4 days (Lai et al., 2005). More recently, a U. S. patient with COVID-19 also shed SARS-CoV-2 RNA in their stool (Holshue et al., 2020) while SARS-CoV-2 RNA was detected in the feces of 59% of 96 infected patients for up to 31 days (Zheng et al., 2020). Thus, a portion of infected humans are capable of shedding novel coronaviruses, including SARS-CoV-2, in their feces, with some for extended (1–4 months) periods. Although there is uncertainty whether SARS-CoV-2 remains infectious in feces, similar coronaviruses, such as SARS-CoV, are infectious in feces, and ACE2 receptors for SARS-CoV-2, are abundant in gastric, duodenal, and rectal epithelia cells in humans (Xiao et al., 2020). This, coupled with the positive detection of SARS-CoV-2 RNA from feces in a large portion of infected patients (Wu et al., 2020b; Xiao et al., 2020; Zheng et al., 2020) suggests that infectious SARS-CoV-2 virions are secreted from gastrointestinal cells into feces (Xiao et al., 2020).
3. Coronavirus survives in sewage and can be introduced into waste water treatment plants
If fecal shedding is substantial in terms of number of infected individuals at focal locations, such as in hospitals, airports, or concentrated housing, then substantial amounts of virus could be deposited into municipal sewage systems (pathways 1 and 2 in Fig. 1). Human coronaviruses (HCoV) can survive for extended periods in the environment, especially aqueous environments (Geller et al., 2012). Survival rates of HCoV in saline ranged from 80 to 100% for 3 days and 30 to 55% for six days (Geller et al., 2012). Using two surrogate HCoVs for SARS CoV, Casanova et al. (2009) found that the viruses remained infectious in water and sewage for 17–22 days at 25 °C and with <1 log10 reduction in infectivity at 4 °C. In addition, SARS-CoV-2 RNA has been detected in untreated wastewater and sewage in sufficient quantities to suggest testing of sewage from municipalities as a viable surveillance strategy (Ahmed et al., 2020; Medema et al., 2020; Wu et al., 2020a). This suggests that water and sewage contaminated with SARS-CoV-2 is a potential vehicle for introduction into the environment, given it can survive the waste water treatment process.
4. Coronavirus survives municipal waste treatment and is introduced into the natural aquatic environment
Once HCoV arrives at waste water treatment plants (WWTP) in sewage (pathway 2 in Fig. 1), it needs to maintain its infectivity through the treatment process to be of concern in the effluent that is ultimately introduced into the natural environment. In general, the transit time for sewage to reach a WWTP is less than one day (Wigginton et al., 2015). While WWTP do reduce virus levels, infective virus is still detected in the effluent from these plants (Wigginton et al., 2015). Based on metagenomics, coronaviruses were detected in 80% of the samples from effluent class B sewage sludge from 5 WWTP in the U.S., which is typically applied to agricultural lands as a soil amendment (Bibby and Peccia, 2013). Although data is lacking for coronaviruses, wastewater treatment prior to disinfection results in 0–2 log10 reduction of infective enteroviruses and 2–>3-log reduction of infective adenoviruses, with infectious virus still being detected in the final effluent from the waste treatment process released into the environment (Simmons and Xagoraraki, 2011; Wigginton et al., 2015). Thus, there is evidence that infectious coronaviruses could pass through the wastewater treatment process and enter natural aquatic systems via WWTP (pathway 3 in Fig. 1).
Even if WWTP completely or mostly eliminated SARS-CoV-2 from their effluent when they operated efficiently, there are numerous opportunities for SARS-CoV-2 to enter the aquatic environment through periodic accidental spills of untreated waste from WWTPs. There are two types of WWTP systems in the U.S. Combined sewer systems (CSS) are the earliest WWTP constructed and collect sewage and storm water runoff through a single pipe system while sanitary sewer systems (SSS) are newer and are separate from municipal storm water systems (U.S. Environmental Protection Agency, 2004). Of the 14,194 WWTP in the U.S., 65.8% are CSS, which are responsible for 850 billion gal of untreated waste being accidentally released each year; SSS are responsible for 3–10 billion gal of spills each year (U. S. Environmental Protection Agency, 2004). Often, these spills coincide with extreme events, such as severe storms. For example, 11 billion gal of untreated (31.3%) and partially treated (68.7%) sewage was released from WWTP into rivers and canals during Hurricane Sandy, mostly in New York City and northern New Jersey (Kenward et al., 2013). Both of these areas are currently experiencing massive COVID-19 outbreaks. During the current COVID-19 pandemic, there have been large sewage spills in outbreak areas. For example in February–March 2020, 16.1 million gal of sewage spilled in two separate accidents near Atlanta, Georgia (Kelly, 2020) and 211 million gal of sewage was released in multiple accidents near Fort Lauderdale, Florida (Brasileiro, 2020). Thus, accidental releases of untreated sewage into the environment are an additional mechanism whereby infectious SARS-CoV-2 could be released into nearby waterways.
5. Bats and other wildlife become infected with coronavirus by drinking contaminated water in the natural environment
Although coronaviruses are assumed to lose infectivity in aquatic environments because of their lipid envelopes, the time for these viruses to reach 90% inactivation (T90) varies considerably, depending on strain, medium characteristics, and temperature (Wigginton et al., 2015). For example, a swine coronavirus has a T90 of 4 days at 25 °C and 24 days at 4 °C in wastewater (Casanova et al., 2009) while HCoV can survive up to 588 days in filtered tap water at 4 °C (Gundy et al., 2009). While, in some circumstances, coronaviruses released into natural bodies of water may become too dilute for uptake by wildlife, there are two mechanisms where dilution of coronavirus would be minimized. The first are if effluent from WWTP is released into a relatively small body of water. For example, one of the WWTP in Fort Collins, Colorado releases its effluent into a slow moving, 8 m-wide irrigation canal that is adjacent to a natural area. In addition, Medema et al. (2020) and Wu et al. (2020a) found high titers of SARS-CoV-2 RNA in sewage at WWTP in both the Netherlands and North America. While not definitive, their results suggest that SARS-CoV-2 is measurable in waste water. A second mechanism is possible bioaccumulation of coronaviruses by molluscs and other aquatic organisms. For example; clams have been found to bioaccumulate avian influenza viruses from the surrounding water (Huyvaert et al., 2012).
Once released into natural aquatic systems, coronaviruses would be available for uptake in wildlife hosts (pathway 4 in Fig. 1). Insectivorous bats (order Chiroptera) are the logical reservoir host for COVID-19 in North America because they were considered the reservoir host for SARS CoV (Banerjee et al., 2019) and are suspected as a host, such as Rhinolophus spp., for SARS-CoV-2 (Lu et al., 2020). In addition, coronaviruses have been detected in feces of North American bats with viral RNA prevalence in shed feces of 17–50%, depending on the species of bat (Dominguez et al., 2007). Other North American wildlife strongly associated with aquatic environments are raccoons (Procyon lotor), which have also been infected with coronaviruses (Martin and Zeidner, 1992). Interestingly, raccoons also feed on aquatic organisms, such as mollusks, that are also potential candidates to bioaccumulate viruses. Based on experimental infections of domestic animals with SARS-CoV-2 (Shi et al., 2020), other wildlife species that may function as wildlife hosts are cats (Felidae) and members of the family Mustelidae (e.g., weasels, skunks, otters, mink, etc.). Examples of mustelids associated with aquatic systems in North America are river otters (Lontra canadensis) and American mink (Neovison vison), which may react similarly to SARS-CoV-2 as the domestic ferrets used in Shi et al. (2020).
However, bats remain the most likely species where spillover of SARS-CoV-2 might occur. Bats drink directly from natural water sources, such as lakes, ponds and slow-flowing streams and rivers, by swooping over the water sources and lapping at the surface (Korine et al., 2016). For some species, use of natural body waters was influenced by proximity to WWTP, where they increased their foraging activity downstream from WWTP relative to upstream (Vaughan et al., 1996; Abbott et al., 2009). This suggests a potential pathway for bats to become infected with HCoV, and presumably SARS-CoV-2, from water sources contaminated with these pathogens from WWTP.
6. Coronavirus spills back from new wildlife hosts to humans
An important consideration in whether SARS-CoV-2 becomes entrenched in North America through a wildlife host is whether spillback from wildlife hosts to humans could occur (pathway 5 in Fig. 1). Spillover into wildlife and subsequent spillback into the human population represents an unmanaged source of SARS-CoV-2. For example, if bats are considered the likely reservoir host for COVID-19, spillback of coronaviruses in North America could occur where bats use buildings for nocturnal roosts or hibernation sites and deposit their feces where humans might encounter them, such as in attics of residences (Voigt et al., 2016). North American bats have been shown to shed coronaviruses in their feces, often at high (up to 50%) prevalence (Dominguez et al., 2007). Airborne transmission of SARS CoV from human fecal matter was considered the primary route of infection of 187 human cases in a housing complex (Yu et al., 2004), indicating that aerosol transmission of HCoV from bat feces to humans is possible.
Although there is still considerable uncertainty about which wildlife species can serve as reservoir hosts for SARS-CoV-2, current research on domestic animals indicates potential candidates in wild cats and mustelids (Shi et al., 2020). Because domestic cats can efficiently replicate SARS-CoV-2 and transmit the virus to naïve cats (Shi et al., 2020), domestic cats represent another mechanism for spillover transmission from wild cats to humans. Another risk associated with the establishment of SARS-CoV-2 in a wildlife host population is the potential for mutation in novel hosts that results in a variant virus (Menachery et al., 2017). Of particular concern, is whether there is the potential for recombination of SARS-CoV-2 with other bat coronaviruses should spillover of SARS-CoV-2 occur in bat populations.
7. Conclusions
While the primary risk associated with the current COVID-19 outbreak appears to be human-to-human transmission of SARS-CoV-2, we believe the existing evidence also supports the plausibility of novel coronaviruses, such as SARS-CoV-2, spilling over to new wildlife hosts through fecal shedding by infected humans and introduction to the natural aquatic environment via the waste water treatment system. In addition, we posit that spillback of novel coronaviruses from the new wildlife hosts is also possible. Thus, considering the current COVID-19 pandemic, wastewater treatment plants and their surrounding environments should come under increased scrutiny for serving as potential areas where spillover into wildlife hosts could occur. This was recently conducted in the Netherlands where SARS-CoV-2 RNA was found in sewage from WWTPs servicing 6 cities and an airport (Medema et al., 2020). Such scrutiny could integrate surveillance of key wildlife species, such as bats and raccoons, which have the potential for acquiring coronaviruses from their aquatic environments. An enhanced surveillance program of identifying SARS-CoV-2 spillover into wildlife would ideally involve 1) identifying “hotspot” municipalities with high human caseloads of COVID-19, 2) identifying areas downstream from WWTP effluent releases, and 3) sampling target wildlife species, such as bats, mustelids, and raccoons, for SARS-CoV-2 antibodies and viral presence. Such an effort could be conducted in conjunction with sampling WWTP effluent as proposed by Medema et al. (2020).
At this stage, a probabilistic risk assessment model would probably not be very informative because of the uncertainties of the mechanisms described here and the need to parameterize the pathways we propose. For example, most detection of coronavirus in fecal and environmental samples have focused on RNA detection through PCR and has, generally, not documented infectivity. We argue that further research into our proposed pathways is warranted, especially because outbreaks of novel, zoonotic pathogens have epidemiological implications beyond just human-to-human transmission.
CRediT authorship contribution statement
Alan B. Franklin: Conceptualization, Visualization, Writing - original draft. Sarah N. Bevins: Conceptualization, Writing - review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The findings and conclusions in this publication are those of the authors and should not be construed to represent any official USDA or U.S. Government determination or policy. This research was supported by the U.S. Department of Agriculture, Animal and Plant Health Inspection Service, Wildlife Services.
References
- Abbott I.M., Sleeman D.P., Harrison S. Bat activity affected by sewage effluent in Irish rivers. Biol. Conserv. 2009;142:2904–2914. [Google Scholar]
- Ahmed W., Angel N., Edson J., Bibby K., Bivins A., O’Brien J.W., Choi P.M., Kitajima M., Simpson S.L., Li J., Tscharke B., Verhagen R., Smith W.J.M., Zaugg J., Dierens L., Hugenholtz P., Thomas K.V., Mueller J.F. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020;138764 doi: 10.1016/j.scitotenv.2020.138764. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A., Kulcsar K., Misra V., Frieman M., Mossman K. Bats and coronaviruses. Viruses. 2019;11:41. doi: 10.3390/v11010041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibby K., Peccia J. Identification of viral pathogen diversity in sewage sludge by metagenome analysis. Environ. Sci. Technol. 2013;47:1945–1951. doi: 10.1021/es305181x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasileiro A. Miami Herald. 2020. Fort Lauderdale’s foul sewage spills have killed fish. There’s likely more damage. [Google Scholar]
- Casanova L., Rutala W.A., Weber D.J., Sobsey M.D. Survival of surrogate coronaviruses in water. Water Res. 2009;43:1893–1898. doi: 10.1016/j.watres.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corman V.M., Albarrak A.M., Omrani A.S., Albarrak M.M., Farah M.E., Almasri M., Muth D., Sieberg A., Meyer B., Assiri A.M., Binger T., Steinhagen K., Lattwein E., Al-Tawfiq J., Müller M.A., Drosten C., Memish Z.A. Viral shedding and antibody response in 37 patients with Middle East respiratory syndrome coronavirus infection. Clin. Infect. Dis. 2015;62:477–483. doi: 10.1093/cid/civ951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez S.R., O’Shea T.J., Oko L.M., Holmes K.V. Detection of group 1 coronaviruses in bats in North America. Emerg. Infect. Dis. 2007;13:1295–1300. doi: 10.3201/eid1309.070491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller C., Varbanov M., Duval R.E. Human coronaviruses: insights into environmental resistance and its influence on the development of new antiseptic strategies. Viruses. 2012;4:3044–3068. doi: 10.3390/v4113044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundy P.M., Gerba C.P., Pepper I.L. Survival of coronaviruses in water and wastewater. Food Environ. Virol. 2009;1:10–14. [Google Scholar]
- Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H., Spitters C., Ericson K., Wilkerson S., Tural A., Diaz G., Cohn A., Fox L., Patel A., Gerber S.I., Kim L., Tong S., Lu X., Lindstrom S., Pallansch M.A., Weldon W.C., Biggs H.M., Uyeki T.M., Pillai S.K. First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huyvaert K.P., Carlson J.S., Bentler K.T., Cobble K.R., Nolte D.L., Franklin A.B. Freshwater clams as bioconcentrators of avian influenza virus in water. Vector-borne Zoon. Dis. 2012;12:904–906. doi: 10.1089/vbz.2012.0993. [DOI] [PubMed] [Google Scholar]
- Ji W., Wang W., Zhao X., Zai J., Li X. Cross-species transmission of the newly identified coronavirus 2019-nCoV. J. Med. Virol. 2020;92:433–440. doi: 10.1002/jmv.25682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly T. CBS46. 2020. DeKalb County officials looking into the cause of two major sewer spills. [Google Scholar]
- Kenward A., Yawitz D., Raja U. Climate Central; Princeton, New Jersey: 2013. Sewage Overflows from Hurricane Sandy. [Google Scholar]
- Korine C., Adams R., Russo D., Fisher-Phelps M., Jacobs D. Bats and water: anthropogenic alteration threatens global bat populations. In: Voigt C.C., Kingston T., editors. Bats in the Anthropocene: Conservation of Bats in a Changing World. Springer International Publishing; Cham: 2016. pp. 215–241. [Google Scholar]
- Lai M.Y.Y., Cheng P.K.C., Lim W.W.L. Survival of severe acute respiratory syndrome coronavirus. Clin. Infect. Dis. 2005;41:e67–e71. doi: 10.1086/433186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung W.K., To K.-f., Chan P.K.S., Chan H.L.Y., Wu A.K.L., Lee N., Yuen K.Y., Sung J.J.Y. Enteric involvement of severe acute respiratory syndrome-associated coronavirus infection. Gastroenterology. 2003;125:1011–1017. doi: 10.1016/j.gastro.2003.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Tang F., Fontanet A., Zhan L., Zhao Q.-M., Zhang P.-H., Wu X.-M., Zuo S.-Q., Baril L., Vabret A., Xin Z.-T., Shao Y.-M., Yang H., Cao W.-C. Long-term SARS coronavirus excretion from patient cohort, China. Emerg. Infect. Dis. 2004;10:1841–1843. doi: 10.3201/eid1010.040297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N., Bi Y., Ma X., Zhan F., Wang L., Hu T., Zhou H., Hu Z., Zhou W., Zhao L., Chen J., Meng Y., Wang J., Lin Y., Yuan J., Xie Z., Ma J., Liu W.J., Wang D., Xu W., Holmes E.C., Gao G.F., Wu G., Chen W., Shi W., Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin H.D., Zeidner N.S. Concomitant Cryptosporidia, coronavirus and parvovirus infection in a raccoon (Procyon lotor) J. Wildl. Dis. 1992;28:113–115. doi: 10.7589/0090-3558-28.1.113. [DOI] [PubMed] [Google Scholar]
- Medema G., Heijnen L., Elsinga G., Italiaander R., Brouwer A. Presence of SARS-Coronavirus-2 in sewage. medRxiv. 2020 doi: 10.1021/acs.estlett.0c00357. (2020.2003.2029.20045880) [DOI] [PubMed] [Google Scholar]
- Menachery V.D., Graham R.L., Baric R.S. Jumping species—a mechanism for coronavirus persistence and survival. Curr. Opin. Virol. 2017;23:1–7. doi: 10.1016/j.coviro.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J., Wen Z., Zhong G., Yang H., Wang C., Huang B., Liu R., He X., Shuai L., Sun Z., Zhao Y., Liu P., Liang L., Cui P., Wang J., Zhang X., Guan Y., Tan W., Wu G., Chen H., Bu Z. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS–coronavirus 2. Science. 2020 doi: 10.1126/science.abb7015. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons F.J., Xagoraraki I. Release of infectious human enteric viruses by full-scale wastewater utilities. Water Res. 2011;45:3590–3598. doi: 10.1016/j.watres.2011.04.001. [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency . U.S. Environmental Protection Agency, Office of Water; Washington, D.C: 2004. Report to Congress: Impacts and Control of CSOs and SSOs. [Google Scholar]
- Vaughan N., Jones G., Harris S. Effects of sewage effluent on the activity of bats (Chiroptera: Vespertilionidae) foraging along rivers. Biol. Conserv. 1996;78:337–343. [Google Scholar]
- Voigt C.C., Phelps K.L., Aguirre L.F., Corrie Schoeman M., Vanitharani J., Zubaid A. Bats and buildings: the conservation of synanthropic bats. In: Voigt C.C., Kingston T., editors. Bats in the Anthropocene: Conservation of Bats in a Changing World. Springer International Publishing; Cham: 2016. pp. 427–462. [Google Scholar]
- Wigginton K.R., Ye Y., Ellenberg R.M. Emerging investigators series: the source and fate of pandemic viruses in the urban water cycle. Environ. Sci. Water Res. Technol. 2015;1:735–746. [Google Scholar]
- Wu F., Xiao A., Zhang J., Gu X., Lee W.L., Kauffman K., Hanage W., Matus M., Ghaeli N., Endo N., Duvallet C., Moniz K., Erickson T., Chai P., Thompson J., Alm E. SARS-CoV-2 titers in wastewater are higher than expected from clinically confirmed cases. medRxiv. 2020 doi: 10.1128/mSystems.00614-20. 2020.2004.2005.20051540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Guo C., Tang L., Hong Z., Zhou J., Dong X., Yin H., Xiao Q., Tang Y., Qu X., Kuang L., Fang X., Mishra N., Lu J., Shan H., Jiang G., Huang X. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol. Hepatol. 2020;5:434–435. doi: 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao F., Tang M., Zheng X., Liu Y., Li X., Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158:1831–1833. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo C., Kaushal S., Yeo D. Enteric involvement of coronaviruses: is faecal-oral transmission of SARS-CoV-2 possible? Lancet Gastroenterol. Hepatol. 2020;5:335–337. doi: 10.1016/S2468-1253(20)30048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu I.T.S., Li Y., Wong T.W., Tam W., Chan A.T., Lee J.H.W., Leung D.Y.C., Ho T. Evidence of airborne transmission of the severe acute respiratory syndrome virus. N. Engl. J. Med. 2004;350:1731–1739. doi: 10.1056/NEJMoa032867. [DOI] [PubMed] [Google Scholar]
- Zheng S., Fan J., Yu F., Feng B., Lou B., Zou Q., Xie G., Lin S., Wang R., Yang X., Chen W., Wang Q., Zhang D., Liu Y., Gong R., Ma Z., Lu S., Xiao Y., Gu Y., Zhang J., Yao H., Xu K., Lu X., Wei G., Zhou J., Fang Q., Cai H., Qiu Y., Sheng J., Chen Y., Liang T. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January–March 2020: retrospective cohort study. BMJ. 2020;369:m1443. doi: 10.1136/bmj.m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]