Highlights
- •
MERS is caused by a single-stranded RNA virus named MERS-corona virus.
- •
Infection with MERS-CoV elicits a type-I and type-II interferon response.
- •
MERS-CoV infected patients show a prominent Th1 and Th17 cytokine profile.
- •
MERS-CoV infection induces the expression of IL-10.
Keywords: MERS-CoV, Cytokines, Interferons, Humans
Abstract
The Middle East respiratory syndrome coronavirus (MERS-CoV) has been recognized as a highly pathogenic virus to humans that infects the respiratory tract and is associated with high morbidity and mortality. Studies in animal models suggest that MERS-CoV infection induces a strong inflammatory response, which may be related to the severity of disease. Data showing the cytokine profiles in humans during the acute phase of MERS-CoV infection are limited. In this study, we have analyzed the profile of cytokine responses in plasma samples from patients with confirmed MERS-CoV infections (n = 7) compared to healthy controls (n = 13). The cytokine profiles, including T helper (Th) 1, Th2 and Th17 responses, were analyzed using cytometric bead array (CBA). A prominent pro-inflammatory Th1 and Th17 response was clearly seen in patients with MERS-CoV infection, with markedly increased concentrations of IFN-γ, TNF-α, IL-15 and IL-17 compared to controls. IL-12 expression levels showed no difference between patients with MERS-CoV infection and the healthy controls despite the significantly increased levels of IFN-α2 and IFN-γ (P < .01). No changes were observed in the levels of IL-2, IL-4, IL-5, IL-13, and TGF-α (P > .05). Our results demonstrate a marked pro-inflammatory cytokine response during the acute phase of MERS-CoV infection in humans.
1. Introduction
The Middle East respiratory syndrome (MERS) was first discovered in 2012 in the Kingdom of Saudi Arabia [1], [2]. MERS is caused by a single-stranded RNA virus named MERS-corona virus (MERS-CoV) that belongs to the genus Beta coronavirus [3]. It is believed that the virus enters the cells through fusion with its plasma membrane. This is achieved when the viral envelop protein S (S protein) binds to dipeptidyl peptidase 4 (DPP4, also known as CD26) as the host receptor, which is abundantly expressed in the lung tissue of mammals [4], [5], [6].
According to January of 2018 data from the European Centre for Disease Prevention and Control, 2122 cases of MERS infection leading to 740 fatalities have been documented worldwide. This indicated a mortality rate of about 39% that was associated with patients who have medical co-morbidities [7], [8], [9]. The majority of cases were reported in the Middle East countries, particularly Saudi Arabia [3], [7]. More recently, a MERS outbreak in the Republic of Korea in 2015 caused a total of 186 confirmed cases with a mortality rate of about 20% [10]. Cases have also been documented in other Asian countries (e.g. Iran, Bangladesh and Malaysia), North Africa (e.g. Egypt, Tunisia, and Algeria), Europe (e.g. United Kingdom, France and Germany) and USA [11], [12], [13], [14], [15], [16], [17].
An earlier study in Saudi Arabia by Briese et al. [18] showed that the whole genome sequence of viruses obtained from humans and camels were almost identical. It was suggested that the virus is transmitted to humans via direct contact with animals, especially dromedary camels in the Middle East [14], [19], [20], [21], [22]. Human-to-human transmission is usually via direct contact with affected individuals and is higher among household and in healthcare settings [23], [24], [25]. Commonly reported symptoms of MERS-CoV infection included cough, fever, breathing difficulty, sore throat, headache, vomiting and diarrhea [7], [26]. Some patients may develop acute respiratory failure and acute kidney injury, which are considered as the major complications of the disease [27], [28].
Laboratory data associated with MERS included leucopaenia, lymphopaenia, disseminated intravascular coagulation, elevated blood creatinine, elevated lactate dehydrogenase and disturbed liver enzyme levels [29], [30], [31]. Viral particles are enriched in respiratory samples while low levels were detected in other body fluids, including blood, urine and stool samples [32]. In this study, we have analyzed the profile of cytokine responses in plasma samples from patients infected with MERS-CoV and show a prominent Th1 and Th17 cytokine profile consistent with a pro-inflammatory response during the acute phase of MERS-CoV infection.
2. Material and methods
2.1. Patients and samples
Blood samples were collected from seven patients (all males, age range between 24 and 49 years) that were admitted to MOH hospitals with an acute onset of “flu-like” symptoms in 2014. Approximately, all patients were presented with fever, chills, cough, and dyspnea. Three patients were suffering from myalgia and two were having gastrointestinal pain. One patient was admitted to the intensive care unit, however, ventilation therapy was not required in his case. Age and gender matched healthy individuals were recruited from the blood bank to serve as the healthy control group.
2.2. Inclusion and exclusion criteria for MERS-CoV patients
Initial diagnosis of MERS-CoV infections was established based on clinical presentation and examination according to the criteria set by World Health Organization recommendations (WHO, 2015). Nasopharyngeal swabs were obtained from patients and healthy controls using viral transport media kits obtained from Hardy Diagnostics (Santa Maria, CA, USA). The diagnosis of MERS-CoV infection was confirmed by real-time polymerase chain reaction (qRT-PCR) using swab specimens as previously described [24]. Patients with other acute or chronic diseases, or infections with other agents were excluded from the study.
2.3. Ethical approvals
The study was approved by the ethical and research committee of the College of Applied Medical Sciences (MLT 2017001), and written informed consents were obtained from all participants.
2.4. Blood samples
Patients with confirmed diagnosis of MERS-CoV infection and who fit the inclusion criteria were included in the study. Venous blood samples from patients were collected directly when the diagnosis was confirmed (approximately 24–48 h after hospital admission) into the vacutainer EDTA tube, and were centrifuged at 4000g for 5 min at 4 °C. The time of sampling relative to the onset of symptoms was estimated to be approximately 4 days based on the information reported by patients to physicians. Plasma samples were collected and stored at −80 °C until used.
2.5. Cytometric Bead Array (CBA) for cytokine measurements
The Cytometric Bead Array (CBA) allows for the simultaneous measurements of several analytes from a single biological sample [33]. CBA array was used in this study to measure multiple cytokines from plasma samples following the manufacturer's instructions (BD Bioscience). The analyzed cytokines included IL-2, IL-4, IL-5, IL-10, IL-12p40, IL-12p70, IL-13, IL-15, IL-17, IFN-α2, IFN-γ, TNF-α and TGF-α. Following flow cytometry (FACScalibur, Becton Dickinson), cytokine concentrations were calculated based on the standard curves using the BD CBA Analysis Software.
2.6. Statistical analysis
All the calculations and statistical analysis were performed using GraphPad Prism statistical software (version 5, USA). Data were expressed as mean ± standard deviation (SD). Two-group comparisons were performed using Mann and Whitney test. P values of <.05 were considered statistically significant.
3. Results
3.1. Infection with MERS-CoV elicits a type-I and type-II interferon response
Significant increases in the levels of interferon gamma (IFN-γ) were observed in the infected patients compared to healthy controls (Fig. 1 , P < .001). IFN-γ is a type-II interferon that is produced by several cell types including NK, NKT and CD4 Th1 cells. IFN-γ interferes with viral replication and plays an important role in both innate and acquired immunity [34]. Notably, there was also a marked increase in IFN-α2 concentration in MERS-COV infected patients, compared to the healthy controls (Fig. 1, p < .01). All the 7 infected patients had a significant increase in IFN-α2 ranging from 26 to 71-fold increase when compared to healthy controls. IFN-α2 is a type-I interferon that serves as a first line of defense against viral infections, which is usually secreted by virally-infected cells to protect neighboring and adjacent cells [35].
Fig. 1.

Changes in the plasma levels of interferons in patients with MERS-CoV infection. Significant increases in the plasma levels of (A) IFN-α2 and (B) IFN-γ. Data are expressed as Mean ± SEM. * indicates significant difference.
3.2. Infection with MERS-CoV is associated with a Th1 and Th17 cytokine profile
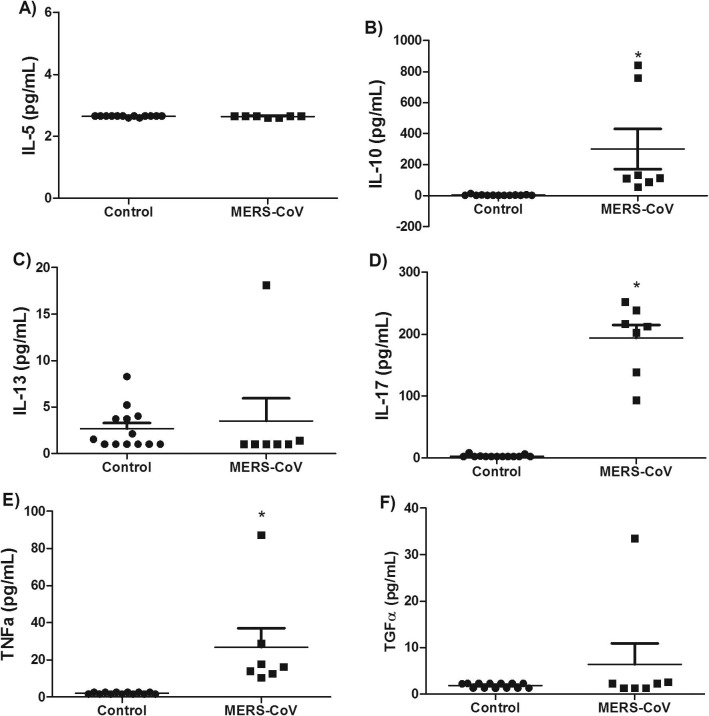
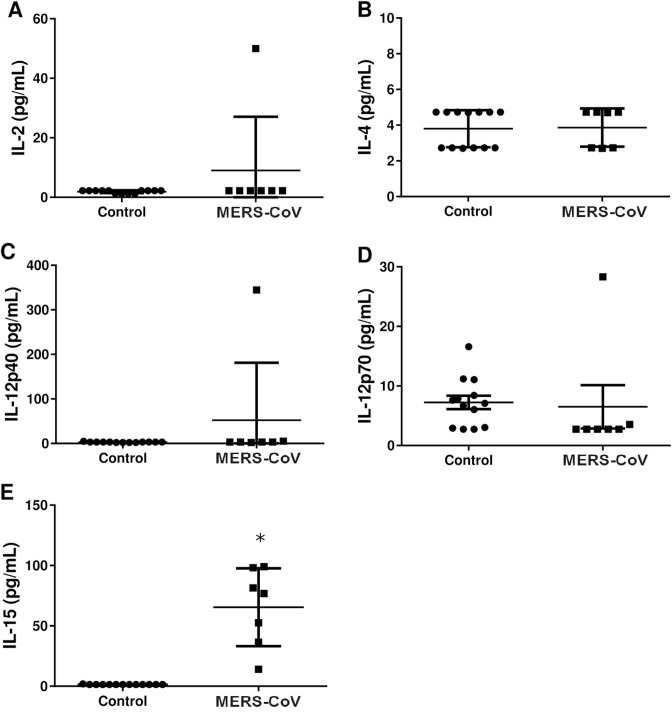
Cytokine profiles, including Th1, Th2 and Th17 cytokines were analyzed in plasma samples from patients diagnosed with an acute phase of MERS-CoV infection as well as from healthy controls. IFN-γ (Fig. 1), TNF-α (Fig. 3 ) and IL-17 (Fig. 3) concentrations were markedly increased in MERS-CoV infected patients compared to healthy controls (p < .01). Interleukin 17 is a pro-inflammatory cytokine produced by T-helper cells in response to STAT3 and NF-κB signaling pathways [36]. All the patients with MERS-CoV infection had a marked increase in IL-17, IFN-γ and TNFα but not IL-12 (IL-12p40 and IL-12p70) when compared to the healthy controls. This finding suggests that MERS-CoV infection in humans activates a Th1 and Th17 pro-inflammatory cytokine profile. On the other hand, there was no significant increase in IL4 (Fig. 2 B), IL5 (Fig. 3A), and IL-13 (Fig. 3C) concentration in MERS-CoV infected patients compared to healthy controls (p > .05). Moreover, significant increase in the levels of TNF-α was detected in plasma samples of patients (P < .01, Fig. 3E) while no change was detected with respect to TGF-α level (P > .05, Fig. 3F). TNF-α is produced by many cells, including macrophages, CD4+ lymphocytes, NK cells, lung-epithelial cells and others [37]. Upon binding to its receptor, TNF-α activates NF-κB, MAPK and apoptotic pathways that interfere with viral replication and assistance viral clearance [37].
Fig. 3.
Levels of selected cytokines in patients with MERS-CoV infection. Plasma levels of (A) IL-5, (B) IL-10, (C) IL-13, (D) IL-17, (E) TNF-α, and (F) TGF-α in patients with MERS-CoV infection. Significant elevations were observed for IL-10, IL-17 and TNF-α while no changes were observed in the levels of IL-5, IL-13 and TGF-α. Data are expressed as Mean ± SEM. * indicates significant difference.
Fig. 2.
Changes in the levels of four α-helix bundle-containing cytokines in patients with MERS-CoV infection. Levels of (A) IL-2, (B) IL-4, (C) IL-12p40, (D) IL-12p70 and (E) IL-15 were measured in plasma samples. Strong elevation only in the levels of IL-15 in all patients (P < .01) was detected. Data are expressed as Mean ± SEM. * indicates significant difference.
3.3. MERS-CoV infection induces the expression of IL-10
IL-10 is an important cytokine that is critical in regulating pro-inflammatory response. In this study, we showed significant increase in IL-10 concentrations in MERS-CoV infected patients compared to healthy controls (Fig. 3). IL-10 belongs to class-II cytokines and has a major anti-inflammatory effect mediated via JAK-STAT pathway [38]. Its production by monocytes and lymphocytes is triggered by pathogenic agents and involve activation of MAPK and NF-κB signaling, and post-transcriptional mechanisms such as control of mRNA stability and microRNAs [39], [40].
4. Discussion
The Middle East respiratory syndrome coronavirus MERS-CoV was first described as an emerging virus in 2012. Since then, the virus has caused a number of outbreaks in many regions of the Middle East. Despite intensive efforts of many researchers, the World Health Organization (WHO) has yet to reach a common consensus on the best treatment approach [41]. This is in part due to the high fatality of the viral infection which causes an acute onset of respiratory symptoms including pneumonia and alveolar destruction [42] leading, in many cases, to death. In this study, we analyzed a panel of 13 inflammatory markers in plasma samples obtained from seven patients diagnosed with MERS-CoV infection. Our results showed a significant elevation in IFN-α2, IFN-γ, TNF-α, IL-15, IL-17 and IL-10 when compared to the healthy group.
In our cytokine array, we examined two interferons (IFN-α2 and IFN-γ) that were significantly elevated in MERS-CoV infected patients. Type-1 interferons are produced by the interaction between certain cellular receptors with viral components including viral genome. Binding of IFN-α2 to its receptor initiates a signaling cascade that leads to an increase in the expression of many downstream signaling targets called interferon-stimulated genes [43]. The products of these genes may play a critical role in inhibition of viral spread into non-infected cells and interference of viral replication in the infected cells [44]. IFN-γ, on the other hand, is known to mediate its effect via the JAK-STAT signaling pathway leading to the subsequent activation of macrophages and NK cells, and expression of specific major histocompatibility complex proteins [45], [46]. A study by Zhou et al. [47] showed that infection of human monocyte-derived macrophages with MERS-CoV induced the expression of IFN-γ and the subsequent expression of major histocompatibility complex class-I genes [47]. In addition, infection of airway epithelial cell lines with MERS-CoV induced the expression of IFN-α2, IFN-γ and TNF-α, possibly through the activation of STAT3 pathway [48]. Elevation of IFN-α2 and IFN-γ was reported from patients infected with MERS-CoV [41]. Since IFN-α2 and IFN-γ were known to promote antigen presentation and the development of a robust antiviral adaptive Th-1 immune response, the elevation of these interferons may be essential to develop immunity against MERS-CoV infection [49]. Consistent with previous findings [47], our results showed a significant elevation in TNF-α levels in response to MERS-CoV infection. Previous studies showed that TNF-α exerts a strong antiviral activity against avian, swine, and human influenza viruses [50].
Our results showed a significant elevation in IL-17A plasma levels in response to MERS-CoV infection, which is in agreement with previous findings from both in vivo[41] and in vitro studies [51]. IL-17 is known to mediate airway remodeling during respiratory infections and allergy by recruiting monocytes and neutrophils to the site of inflammation and enhancing the production of other cytokines and chemokines such as IL-6, IL-1β, TGF-β, TNF-α, IL-8 and MCP-1 [52].
Our results also showed a significant increase in the plasma levels of IL-10 in infected patients. The IL-10 expression has been shown to be associated with persistent viral infections such as HIV, HCV and HBV [53]. Elevation of IL-10 was reported in patients infected with MERS-CoV [41]. The increase in levels of IL-10 after infection with MERS-CoV may play a role in host immune regulation in inhibiting the proinflammatory response induced by the infection.
The notion that STAT3 may antagonize the expression of certain NF-κB activated genes is not without precedence. It has been previously shown that IL-10 and IL-17 not only act as STAT3 activators, but are also upregulated by its expression [54], [55]. When induced, STAT3 selectively inhibits NF-κB activated genes, such as IL-12, required for the successful activation of Th1 immune response [56]. The exact role played by STAT3 in viral infections is still to be elucidated. Some viral infections, such as HBV [57] and HCV [58], promote STAT3-mediated gene expression; while other viral infections, such as influenza A virus [59] and SARS CoV [60], disrupt STAT3-mediated gene expression.
Similar to other pro-inflammatory cytokines, a significant increase of IL-15 in plasma levels from MERS-CoV infected patients was observed. IL-15 is usually secreted by mononuclear phagocytes, monocytes, and dendritic cells in response to infection by viruses [61]. The expression of IL-15 enhances proliferation of NK and CD8+ T cells that mediate the elimination of virally infected cells [62]. The levels of IL-4, IL-5 and IL-13 were not affected by MERS-CoV infection. This is consistent with the assumption that MERS-CoV infection does not activate a significant Th2 type response.
Surprisingly, patients with MERS-CoV infection did not show a significant increase in the plasma levels of IL-12. IL-12 is involved in natural killer cell and T cell activation [63]. Previous in vitro experiments showed that IL-12 expression was elevated after MERS-CoV infection through IRF3 activation [64], which is a known regulator of STAT3 [65].
Among the limitations of the current investigation is that MERS-CoV virus is mainly a respiratory disease and lung immune response is known to be compartmentalized. Thus, assessment of host response in sera of infected patients may not be representative of host response against the MERS-CoV in the airways. In addition, patients were not followed and therefore, clinical outcomes of the infection were not assessed. Moreover, the plasma cytokine levels were measured in a single blood sample and therefore, it is impossible to know whether peak cytokine levels were measured. Future studies that include examining broncho-alveolar lavage to get a better representation of host immune are strongly recommended. In addition, correlating changes in cytokine profile with clinical outcomes of the infection would enhance the expected knowledge from such studies. The results of plasma cytokine need to be confirmed in a larger sample with multiple sampling times to be able to catch peak cytokine levels.
In conclusion, we report an increase in the plasma levels of IFN-α2, IFN-γ, TNF-α, IL-10, IL-15 and IL-17 during MERS-CoV infection. These findings would serve the scientific community in delineating the pathogenic approach by which MERS-CoV manifests its lethal complications. Overall, our findings may have important implications for management strategies as well as future therapeutic approaches against this virus.
Acknowledgments
Acknowledgment
The authors would like to thank Dr. Hisham Fakher and Dr. Waad Fakhouri for their valuable contribution in RT-PCR data. The study is supported by Taibah University.
Declaration of interest
Authors have nothing to declare.
References
- 1.Mackay I.M., Arden K.E. MERS coronavirus: diagnostics, epidemiology and transmission. Virol. J. 2015;12:222. doi: 10.1186/s12985-015-0439-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thomas C. MERS-CoV: where are we now? Ann. Acad. Med. Singapore. 2015;44:155–156. [PubMed] [Google Scholar]
- 3.Almaghrabi R.S., Omrani A.S. Middle East respiratory syndrome coronavirus (MERS-CoV) infection. Brit. J. Hospital Med. 2017;78:23–26. doi: 10.12968/hmed.2017.78.1.23. [DOI] [PubMed] [Google Scholar]
- 4.Liu W.J., Zhao M., Liu K., Xu K., Wong G., Tan W. T-cell immunity of SARS-CoV: implications for vaccine development against MERS-CoV. Antiviral Res. 2017;137:82–92. doi: 10.1016/j.antiviral.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu G., Hu Y., Wang Q., Qi J., Gao F., Li Y. Molecular basis of binding between novel human coronavirus MERS-CoV and its receptor CD26. Nature. 2013;500:227–231. doi: 10.1038/nature12328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song W., Wang Y., Wang N., Wang D., Guo J., Fu L. Identification of residues on human receptor DPP4 critical for MERS-CoV binding and entry. Virology. 2014;471–473:49–53. doi: 10.1016/j.virol.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Tawfiq J.A., Memish Z.A. Emerging respiratory viral infections: MERS-CoV and influenza. Lancet Respirat. Med. 2014;2:23–25. doi: 10.1016/S2213-2600(13)70255-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Badawi A., Ryoo S.G. Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS-CoV): a systematic review and meta-analysis. Int. J. Infect. Diseases: IJID: official publication of the International Society for Infectious Diseases. 2016;49:129–133. doi: 10.1016/j.ijid.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banik G.R., Khandaker G., Rashid H. Middle East respiratory syndrome coronavirus “MERS-CoV”: current knowledge gaps. Paediat. Respir. Rev. 2015;16:197–202. doi: 10.1016/j.prrv.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsieh Y.H. 2015 Middle East Respiratory Syndrome Coronavirus (MERS-CoV) nosocomial outbreak in South Korea: insights from modeling. PeerJ. 2015;3:e1505. doi: 10.7717/peerj.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bermingham A., Chand M.A., Brown C.S., Aarons E., Tong C., Langrish C. Severe respiratory illness caused by a novel coronavirus, in a patient transferred to the United Kingdom from the Middle East, September 2012. Euro surveillance: bulletin Europeen sur les maladies transmissibles =European communicable disease bulletin. 2012;17:20290. [PubMed] [Google Scholar]
- 12.Fisman D.N., Tuite A.R. The epidemiology of MERS-CoV. Lancet Infect. Diseases. 2014;14:6–7. doi: 10.1016/S1473-3099(13)70283-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gautret P., Gray G.C., Charrel R.N., Odezulu N.G., Al-Tawfiq J.A., Zumla A. Emerging viral respiratory tract infections–environmental risk factors and transmission. The Lancet Infect. Diseases. 2014;14:1113–1122. doi: 10.1016/S1473-3099(14)70831-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hemida M.G., Elmoslemany A., Al-Hizab F., Alnaeem A., Almathen F., Faye B. Dromedary camels and the transmission of Middle East Respiratory Syndrome Coronavirus (MERS-CoV) Transbound. Emerg. Diseases. 2015 doi: 10.1111/tbed.12401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.A. Mailles, K. Blanckaert, P. Chaud, S. van der Werf, B. Lina, V. Caro, et al., First cases of Middle East Respiratory Syndrome Coronavirus (MERS-CoV) infections in France, investigations and implications for the prevention of human-to-human transmission, France, May 2013. Euro surveillance: bulletin Europeen sur les maladies transmissibles = European Communicable Disease Bulletin 18 (2013). [PubMed]
- 16.J. Premila Devi, W. Noraini, R. Norhayati, C. Chee Kheong, A.S. Badrul, S. Zainah, et al., Laboratory-confirmed case of Middle East respiratory syndrome coronavirus (MERS-CoV) infection in Malaysia: preparedness and response, April 2014. Euro surveillance: bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin 19 (2014). [DOI] [PubMed]
- 17.Regan J.J., Jungerman M.R., Lippold S.A., Washburn F., Roland E., Objio T. Tracing airline travelers for a public health investigation: Middle East Respiratory Syndrome Coronavirus (MERS-CoV) Infection in the United States, 2014. Public Health Reports. 2016;131:552–559. doi: 10.1177/0033354916662213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.T. Briese, N. Mishra, K. Jain, I.S. Zalmout, O.J. Jabado, W.B. Karesh, et al., Middle East respiratory syndrome coronavirus quasispecies that include homologues of human isolates revealed through whole-genome analysis and virus cultured from dromedary camels in Saudi Arabia, mBio 5 (2014) e01146–e01214. [DOI] [PMC free article] [PubMed]
- 19.Omrani A.S., Al-Tawfiq J.A., Memish Z.A. Middle East respiratory syndrome coronavirus (MERS-CoV): animal to human interaction. Pathogens and Global Health. 2015;109:354–362. doi: 10.1080/20477724.2015.1122852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reeves T., Samy A.M., Peterson A.T. MERS-CoV geography and ecology in the Middle East: analyses of reported camel exposures and a preliminary risk map. BMC Res. Notes. 2015;8:801. doi: 10.1186/s13104-015-1789-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reperant L.A., Osterhaus A.D. Dromedary MERS-CoV replicates in human respiratory tissues. The Lancet Respir. Med. 2014;2:779–781. doi: 10.1016/S2213-2600(14)70184-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yusof M.F., Eltahir Y.M., Serhan W.S., Hashem F.M., Elsayed E.A., Marzoug B.A. Prevalence of Middle East respiratory syndrome coronavirus (MERS-CoV) in dromedary camels in Abu Dhabi Emirate, United Arab Emirates. Virus Genes. 2015;50:509–513. doi: 10.1007/s11262-015-1174-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cauchemez S., Nouvellet P., Cori A., Jombart T., Garske T., Clapham H. Unraveling the drivers of MERS-CoV transmission. Proc. Natl. Acad. Sci. USA. 2016;113:9081–9086. doi: 10.1073/pnas.1519235113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohd H.A., Memish Z.A., Alfaraj S.H., McClish D., Altuwaijri T., Alanazi M.S. Predictors of MERS-CoV infection: a large case control study of patients presenting with ILI at a MERS-CoV referral hospital in Saudi Arabia. Travel Med. Infect. Disease. 2016;14:464–470. doi: 10.1016/j.tmaid.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zumla A., Hui D.S. Infection control and MERS-CoV in health-care workers. Lancet. 2014;383:1869–1871. doi: 10.1016/S0140-6736(14)60852-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arabi Y.M., Harthi A., Hussein J., Bouchama A., Johani S., Hajeer A.H. Severe neurologic syndrome associated with Middle East respiratory syndrome corona virus (MERS-CoV) Infection. 2015;43:495–501. doi: 10.1007/s15010-015-0720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cha R.H., Joh J.S., Jeong I., Lee J.Y., Shin H.S., Kim G. Renal complications and their prognosis in Korean patients with Middle East Respiratory Syndrome-Coronavirus from the central MERS-CoV designated hospital. J. Kor. Med. Sci. 2015;30:1807–1814. doi: 10.3346/jkms.2015.30.12.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khalid I., Alraddadi B.M., Dairi Y., Khalid T.J., Kadri M., Alshukairi A.N. Acute management and long-term survival among subjects with severe Middle East Respiratory Syndrome coronavirus Pneumonia and Ards. Respir. Care. 2016;61:340–348. doi: 10.4187/respcare.04325. [DOI] [PubMed] [Google Scholar]
- 29.Assiri A., McGeer A., Perl T.M., Price C.S., Al Rabeeah A.A., Cummings D.A. Hospital outbreak of Middle East respiratory syndrome coronavirus. N. Engl. J. Med. 2013;369:407–416. doi: 10.1056/NEJMoa1306742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ko J.H., Park G.E., Lee J.Y., Lee J.Y., Cho S.Y., Ha Y.E. Predictive factors for pneumonia development and progression to respiratory failure in MERS-CoV infected patients. J. Infect. 2016;73:468–475. doi: 10.1016/j.jinf.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sherbini N., Iskandrani A., Kharaba A., Khalid G., Abduljawad M., Al-Jahdali H. Middle East respiratory syndrome coronavirus in Al-Madinah City, Saudi Arabia: demographic, clinical and survival data. J. Epidemiol. Global Health. 2017;7:29–36. doi: 10.1016/j.jegh.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee H., Ki C.S., Sung H., Kim S., Seong M.W., Yong D. Guidelines for the laboratory diagnosis of Middle East respiratory syndrome coronavirus in Korea. Infect. Chemother. 2016;48:61–69. doi: 10.3947/ic.2016.48.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morgan E., Varro R., Sepulveda H., Ember J.A., Apgar J., Wilson J. Cytometric bead array: a multiplexed assay platform with applications in various areas of biology. Clin. Immunol. 2004;110:252–266. doi: 10.1016/j.clim.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 34.Gattoni A., Parlato A., Vangieri B., Bresciani M., Derna R. Interferon-gamma: biologic functions and HCV terapy (type I/II) (2 of 2 parts) La Clinica terapeutica. 2006;157:457–468. [PubMed] [Google Scholar]
- 35.Tovey M.G., Lallemand C. Safety, tolerability, and immunogenicity of interferons. Pharmaceuticals. 2010;3:1162–1186. doi: 10.3390/ph3041162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manni M.L., Robinson K.M., Alcorn J.F. A tale of two cytokines: IL-17 and IL-22 in asthma and infection. Expert Rev. Respir. Med. 2014;8:25–42. doi: 10.1586/17476348.2014.854167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herbein G., O'Brien W.A. Tumor necrosis factor (TNF)-alpha and TNF receptors in viral pathogenesis. Proc. Soc. Exper. Biol. Med. Soc. Exper. Biol. Med. 2000;223:241–257. doi: 10.1177/153537020022300305. [DOI] [PubMed] [Google Scholar]
- 38.Dumoutier L., Renauld J.C. Viral and cellular interleukin-10 (IL-10)-related cytokines: from structures to functions. Eur. Cytokine Network. 2002;13:5–15. [PubMed] [Google Scholar]
- 39.Carey A.J., Tan C.K., Ulett G.C. Infection-induced IL-10 and JAK-STAT: a review of the molecular circuitry controlling immune hyperactivity in response to pathogenic microbes. Jak-Stat. 2012;1:159–167. doi: 10.4161/jkst.19918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang L., Li G., Yao Z.Q., Moorman J.P., Ning S. MicroRNA regulation of viral immunity, latency, and carcinogenesis of selected tumor viruses and HIV. Rev. Med. Virol. 2015;25:320–341. doi: 10.1002/rmv.1850. [DOI] [PubMed] [Google Scholar]
- 41.Faure E., Poissy J., Goffard A., Fournier C., Kipnis E., Titecat M. Distinct immune response in two MERS-CoV-infected patients: can we go from bench to bedside? PloS One. 2014;9:e88716. doi: 10.1371/journal.pone.0088716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zaki A.M., Van Boheemen S., Bestebroer T.M., Osterhaus A.D., Fouchier R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. New Engl. J. Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 43.Zaritsky L.A., Bedsaul J.R., Zoon K.C. Virus multiplicity of infection affects type I interferon subtype induction profiles and interferon-stimulated genes. J. Virol. 2015;89:11534–11548. doi: 10.1128/JVI.01727-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim I.W., Hwang J.Y., Kim S.K., Kim J.K., Park H.S. Interferon-stimulated genes response in endothelial cells following Hantaan virus infection. J. Kor. Med. Sci. 2007;22:987–992. doi: 10.3346/jkms.2007.22.6.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kroetz D.N., Allen R.M., Schaller M.A., Cavallaro C., Ito T., Kunkel S.L. Type I interferon induced epigenetic regulation of macrophages suppresses innate and adaptive immunity in acute respiratory viral infection. PLoS Pathogens. 2015;11:e1005338. doi: 10.1371/journal.ppat.1005338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stubblefield Park S.R., Widness M., Levine A.D., Patterson C.E. T cell-, interleukin-12-, and gamma interferon-driven viral clearance in measles virus-infected brain tissue. J. Virol. 2011;85:3664–3676. doi: 10.1128/JVI.01496-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou J., Chu H., Li C., Wong B.H., Cheng Z.S., Poon V.K. Active replication of Middle East respiratory syndrome coronavirus and aberrant induction of inflammatory cytokines and chemokines in human macrophages: implications for pathogenesis. J. Infect. Diseases. 2014;209:1331–1342. doi: 10.1093/infdis/jit504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Selinger C., Tisoncik-Go J., Menachery V.D., Agnihothram S., Law G.L., Chang J. Cytokine systems approach demonstrates differences in innate and pro-inflammatory host responses between genetically distinct MERS-CoV isolates. BMC Genom. 2014;15:1161. doi: 10.1186/1471-2164-15-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.DeDiego M.L., Nieto-Torres J.L., Jimenez-Guardeno J.M., Regla-Nava J.A., Castano-Rodriguez C., Fernandez-Delgado R. Coronavirus virulence genes with main focus on SARS-CoV envelope gene. Virus Res. 2014;194:124–137. doi: 10.1016/j.virusres.2014.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seo S.H., Webster R.G. Tumor necrosis factor alpha exerts powerful anti-influenza virus effects in lung epithelial cells. J. Virol. 2002;76:1071–1076. doi: 10.1128/JVI.76.3.1071-1076.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.L. Josset, V.D. Menachery, L.E. Gralinski, S. Agnihothram, P. Sova, V.S. Carter, et al., Cell host response to infection with novel human coronavirus EMC predicts potential antivirals and important differences with SARS coronavirus, mBio 4 (2013) e00165–e00213. [DOI] [PMC free article] [PubMed]
- 52.Jin W., Dong C. IL-17 cytokines in immunity and inflammation. Emerg. Microbes Infect. 2013;2:e60. doi: 10.1038/emi.2013.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Blackburn S.D., Wherry E.J. IL-10, T cell exhaustion and viral persistence. Trends Microbiol. 2007;15:143–146. doi: 10.1016/j.tim.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 54.Ogura H., Murakami M., Okuyama Y., Tsuruoka M., Kitabayashi C., Kanamoto M. Interleukin-17 promotes autoimmunity by triggering a positive-feedback loop via interleukin-6 induction. Immunity. 2008;29:628–636. doi: 10.1016/j.immuni.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 55.Donnelly R.P., Dickensheets H., Finbloom D.S. The interleukin-10 signal transduction pathway and regulation of gene expression in mononuclear phagocytes. J. Interferon Cytokine Res. 1999;19:563–573. doi: 10.1089/107999099313695. [DOI] [PubMed] [Google Scholar]
- 56.Darnell J.E., Jr, Kerr I.M., Stark G.R. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science-AAAS-weekly paper edition-including guide to scientific information. 1994;264:1415–1420. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 57.Waris G., Siddiqui A. Interaction between STAT-3 and HNF-3 leads to the activation of liver-specific hepatitis B virus enhancer 1 function. J. Virol. 2002;76:2721–2729. doi: 10.1128/JVI.76.6.2721-2729.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stevenson N.J., Bourke N.M., Ryan E.J., Binder M., Fanning L., Johnston J.A. Hepatitis C virus targets the interferon-α JAK/STAT pathway by promoting proteasomal degradation in immune cells and hepatocytes. FEBS Lett. 2013;587:1571–1578. doi: 10.1016/j.febslet.2013.03.041. [DOI] [PubMed] [Google Scholar]
- 59.Jia D., Rahbar R., Chan R.W., Lee S.M., Chan M.C., Wang B.X. Influenza virus non-structural protein 1 (NS1) disrupts interferon signaling. PloS One. 2010;5:e13927. doi: 10.1371/journal.pone.0013927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mizutani T., Fukushi S., Murakami M., Hirano T., Saijo M., Kurane I. Tyrosine dephosphorylation of STAT3 in SARS coronavirus-infected Vero E6 cells. FEBS Lett. 2004;577:187–192. doi: 10.1016/j.febslet.2004.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ali A.K., Nandagopal N., Lee S.H. IL-15-PI3K-AKT-mTOR: a critical pathway in the life journey of natural killer cells. Front. Immunol. 2015;6:355. doi: 10.3389/fimmu.2015.00355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Adib-Conquy M., Scott-Algara D., Cavaillon J.M., Souza-Fonseca-Guimaraes F. TLR-mediated activation of NK cells and their role in bacterial/viral immune responses in mammals. Immunol Cell Biol. 2014;92:256–262. doi: 10.1038/icb.2013.99. [DOI] [PubMed] [Google Scholar]
- 63.Gee K., Guzzo C., Che Mat N.F., Ma W., Kumar A. The IL-12 family of cytokines in infection, inflammation and autoimmune disorders. Inflam. Allergy Drug Targets. 2009;8:40–52. doi: 10.2174/187152809787582507. [DOI] [PubMed] [Google Scholar]
- 64.Chu H., Zhou J., Wong B.H.-Y., Li C., Cheng Z.-S., Lin X. Productive replication of Middle East respiratory syndrome coronavirus in monocyte-derived dendritic cells modulates innate immune response. Virology. 2014;454:197–205. doi: 10.1016/j.virol.2014.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lu R., Moore P.A., Pitha P.M. Stimulation of IRF-7 gene expression by tumor necrosis factor α requirement for NFκB transcription factor and gene accessibility. J. Biolog. Chem. 2002;277:16592–16598. doi: 10.1074/jbc.M111440200. [DOI] [PubMed] [Google Scholar]