Abstract
Adenoviruses have been identified in humans and a wide range of vertebrate animals, but not previously from the polar region. Here, we report the entire 26,340-bp genome of a novel adenovirus, detected by PCR, in tissues of six of nine South Polar skuas (Catharacta maccormicki), collected in Lake King Sejong, King George Island, Antarctica, from 2007 to 2009. The DNA polymerase, penton base, hexon and fiber genes of the South Polar skua adenovirus (SPSAdV) exhibited 68.3%, 75.4%, 74.9% and 48.0% nucleotide sequence similarity with their counterparts in turkey hemorrhagic enteritis virus. Phylogenetic analysis based on the entire genome revealed that SPSAdV belonged to the genus Siadenovirus, family Adenoviridae. This is the first evidence of a novel adenovirus, SPSAdV, from a large polar seabird (family Stercorariidae) in Antarctica.
Keywords: Adenovirus, Full genome sequence, South Polar skua, Catharacta maccormicki, Antarctica
Introduction
Adenoviruses have linear, non-segmented, double-stranded DNA genomes, which range between 26 and 43 kb and are generally characteristic of each genus (Davison et al., 2003, Klempa et al., 2009, Kovács and Benkö, 2011, Mase et al., 2009).
The family Adenoviridae is comprised of five genera: Mastadenovirus, Aviadenovirus, Atadenovirus, Siadenovirus and Ichtadenovirus (Houng et al., 2006, Kovács and Benkö, 2011, Kovács et al., 2010, Lehmkuhl and Hobbs, 2008, Wellehan et al., 2004), which infect a wide range of vertebrate species (Davison et al., 2003, Morrison et al., 1997). Mastadenovirus has been identified in mammals, including human, sea lion, canine, bovine, porcine, murine and bat (Aggarwal and Mittal, 2000, Goldstein et al., 2011, Klempa et al., 2009, Kovács et al., 2004, Li et al., 2010, Morrison et al., 1997, Rusvai et al., 2000). Aviadenovirus contains falcon and other fowl adenoviruses (Davison et al., 2000). Atadenovirus has been found in snake, marsupial and ruminants (Dan et al., 1998, Farkas et al., 2008, Thomson et al., 2002). Siadenovirus has been detected in frog, raptor and turkey (Beach et al., 2009, Davison and Harrach, 2002, Davison et al., 2000, Kovács and Benkö, 2009). A new genus, Ichtadenovirus, has been identified recently in fish (Benkö et al., 2005).
Typically, adenovirus infection in most species is characterized by enteritis and respiratory disease (Beach et al., 2009, Russell, 2009, Rux and Burnett, 2004, Schrenzel et al., 2005). However, other clinical manifestations have been observed. For example, turkey hemorrhagic enteritis virus (THEV) causes inclusion body hepatitis, depression, splenomegaly, immunosuppression and death (Beach et al., 2009, Jucker et al., 1996, Pitcovski et al., 1998); falcon adenovirus also causes hepatitis (Schrenzel et al., 2005); and agamid adenovirus infection can be subclinical or lethal (Wellehan et al., 2004).
An understanding of virus diversity in wildlife provides epidemiological and ecological information about potential pathogens and may lead to the identification of newly emerging microbial threats. A previous study reported that some Antarctic avifauna is infected with various viruses, which may have been spread by Antarctic birds (Austin and Webster, 1993, Stannard et al., 1998). The South Polar skua (Catharacta maccormicki, previously known as Stercorarius maccormicki), which migrates for their breeding season (Yogui and Sericano, 2009), is an important top predator, exhibiting piratical behavior throughout the year.
In the present study, we examined various organs from carcasses of South Polar skuas, collected in Antarctica during 2007 to 2009, for evidence of adenovirus infection. The genetic and phylogenetic analyses of a newfound South Polar skua adenovirus (SPSAdV) are reported.
Results
Identification of SPSAdV
Initially, the DNA polymerase (pol) gene of a previously unknown adenovirus was amplified by PCR from the kidney of a South Polar skua (SPS T03). Using newly designed primers based on the obtained sequence, the full genome of the novel adenovirus (SPSAdV) was extended from the left-end inverted terminal repeat (ITR) region to the right-end ITR region. The entire viral DNA genome was 26,340 bp and encoded 24 adenoviral genes. The complete nucleotide sequence of SPSAdV was deposited in GenBank (accession number HM585353). The G + C content was found to be 34.2%.
The pol, penton and hexon genes of SPSAdV from four other skuas (SPS T01, SPS T02, SPS T06 and SPS T09) were also sequenced (Table 2 ), while from SPS T08 only the penton gene was sequenced from the liver, suggesting lower virus concentration. Nucleotide sequences of the pol, penton and/or hexon genes of SPSAdV identified in the six skuas were identical, with no evidence of polymorphism. Since separate sterile instruments were used in collecting tissues from each animal under BSL-2 containment, it is unlikely that the identical sequences were due to cross contamination.
Table 2.
Detection of South Polar skua adenovirus in various tissues by PCR.
| Animal no. | Accession no. | Genome/gene(s) | Detected tissue |
|---|---|---|---|
| SPS T03 | HM585353 | Complete (26,340 bp) | Heart, Lung, Liver, Kidney, Intestine, Trachea |
| SPS T01 | HM585354 | Polymerase, Penton, Hexon | Liver, Kidney, Intestine |
| SPS T02 | HM585355 | Polymerase, Penton, Hexon | Kidney, Trachea |
| SPS T06 | HM585356 | Polymerase, Penton, Hexon | Lung, Liver, Kidney |
| SPS T08 | HM585357 | Penton | Liver |
| SPS T09 | HM585358 | Polymerase, Penton, Hexon | Lung, Kidney, Intestine |
Sequence analysis
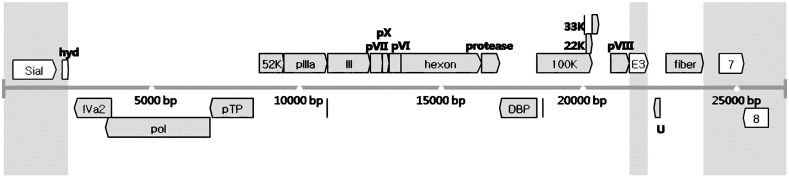
The positions and coding directions of the 24 genes and open reading frames (ORF) are shown in the schematic genome map (Fig. 2 ). The locations, as well as lengths of each gene (nucleotide and amino acid) and their G + C content, are indicated in Table 3 . The ITR regions were located on left and right ends. IVa2, polymerase, protein precursor (pTP), DNA binding protein (DBP), U exon and ORF8 were transcribed leftward, and sialidase, ORF4, 52K, pIIIa, III (penton), pVII, pX, pVI, hexon, protease, 100K, 22K, 33K, pVIII, E3 region, fiber and ORF7 were transcribed rightward.
Fig. 2.
Genome map of South Polar skua adenovirus. The central horizontal line represents the double-stranded DNA marked at 5-kb intervals. Gray blocks show genus-common genes that can be found in every Adenoviridae genus. Sial (sialidase), hyd (hydrophobic), E3, ORF 7 and ORF 8 are genes that can be found only in Siadenovirus. Putative proteins were determined by aligning sequences of other siadenoviruses.
Table 3.
Size and position of genes of South Polar skua adenovirus.
| Gene | Strand | Location | Nucleotides | Amino acids | G + C content (%) |
|---|---|---|---|---|---|
| ITR | Both | 1–30 | 30 | – | 40.00 |
| Sialidase | r | 331–2001 | 1671 | 556 | 38.06 |
| ORF4 | r | 2028–2351 | 324 | 107 | 44.75 |
| IVa2 | l | 2402–3499 | 1098 | 365 | 29.69 |
| DNA pol | l | 3492–6827 | 3336 | 1111 | 31.71 |
| pTP | l | 6824–8600; 11,001–11,020 | 1797 | 598 | 34.11 |
| 52K | r | 8628–9503 | 876 | 291 | 33.90 |
| pIIIa | r | 9493–11,004 | 1512 | 503 | 32.27 |
| III | r | 11,026–12,375 | 1350 | 449 | 33.26 |
| pVII | r | 12,375–12,785 | 411 | 136 | 46.96 |
| pX | r | 12,792–12,968 | 177 | 58 | 36.72 |
| pVI | r | 12,987–13,652 | 666 | 221 | 37.84 |
| Hexon | r | 13,661–16,393 | 2733 | 910 | 34.36 |
| Protease | r | 16,393–17,001 | 609 | 202 | 30.54 |
| DBP | l | 17,034–18,131; 18,208–18,240 | 1131 | 376 | 38.73 |
| 100K | r | 18,284–20,380 | 2097 | 698 | 33.43 |
| 33K | r | 20,274–20,376; 20,601–20,851 | 354 | 117 | 29.38 |
| 22K | r | 20,274–20,561 | 288 | 95 | 35.42 |
| pVIII | r | 20,944–21,579 | 636 | 211 | 43.24 |
| E3 | r | 21,425–22,315 | 891 | 296 | 30.98 |
| U exon | l | 22,326–22,592 | 267 | 88 | 33.33 |
| Fiber | r | 22,600–23,988 | 1389 | 462 | 33.05 |
| ORF 7 | r | 24,426–25,088 | 663 | 220 | 36.50 |
| ORF 8 | l | 25,103–25,600 | 498 | 165 | 37.95 |
The length of the ITR region differed depending on the adenovirus species. For example, the ITR of THEV (AY849321) was 40 bp, whereas that of HAdV-1 (AC000017) was 103 bp. By contrast, the ITR of SPSAdV was 31 bp, whereas the lengths of the 5′ and 3′ terminal ends were the same as those of other species.
The sialidase of SPSAdV, located immediately downstream of the ITR region, was composed of ORF1, ORF2 and ORF3. In SPSAdV, ORF4, located immediately next to the sialidase, was identified as hydrophobic, as in RAdV-1, THEV and FrAdV-1. IVa2, a delayed early gene located downstream of the polymerase gene, was slightly shorter than that in the avirulent turkey enteritis virus (1104 bp) and its G + C content of 29.69% was lower than that of other genes. The E2 region, containing the genes for DNA polymerase, pTP and DBP, consisted of two cleavage sites. The penton base, encoding a major capsid protein of adenovirus, was located between the pIIIa and pVII. And the hexon gene, encoding a capsid protein with a penton base and a fiber knob, had a G + C content of 34.36%.
The protease gene encoded one of the most conserved proteins among all adenovirus genes (Russell, 2009, Weber, 2007). The length of the E3 gene was 891 bp, and the 1389-nucleotide fiber gene encoded a 462-amino acid capsid protein, which was located between the U exon and ORF 7 (22,600–23,988) and transcribed in the rightward direction. ORF7 and ORF8 were genus specific, existing only in Siadenovirus.
Phylogenetic analysis
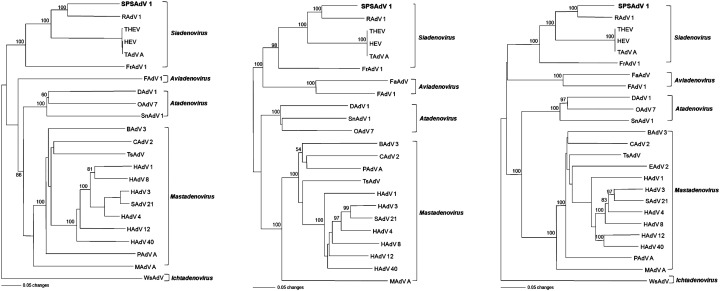
The viral genome and phylogenetic analysis showed that SPSAdV belonged to genus Siadenovirus in the family Adenoviridae (Fig. 3 ). At the nucleotide level, the SPSAdV pol, penton base and hexon genes exhibited somewhat higher sequence similarity of 73.8%, 79.2% and 77.5% with RAdV-1 than with THEV (68.3%, 75.4% and 74.9%) (Table 4 ).
Fig. 3.
Phylogenetic trees, based on the entire amino acid sequences of the polymerase (left), penton base (middle), hexon (right) genes, generated by the neighbor-joining method. Phylogenetic relationships of SPSAdV are shown with raptor adenovirus 1 (RAdV-1, EU715130), avirulent turkey hemorrhagic enteritis virus (THEV, AY849321), hemorrhagic enteritis virus (HEV, AF074946), turkey adenovirus A (TAdV-A, AC000016), frog adenovirus 1 (FrAdV-1, AF224336), psittacine adenovirus (PsAdV, pol, EU056825; hexon, EU627198), Sulawesi tortoises adenovirus (STAdV, EU056826), great tit adenovirus (GTAdV, FJ849795), fowl adenovirus 1 (FAdV-1, U46933), falcon adenovirus (FaAdV, AY683541), duck adenovirus 1 (DAdV-1, Y09598), snake adenovirus 1 (SnAdV-1, DQ106414), ovine adenovirus 7 (OAdV-7, OAU40839), bovine adenovirus 3 (BAdV-3, AF030154), canine adenovirus 2 (CAdV-2, AC000020), porcine adenovirus A (PAdV-A, NC_005869), tree shrew adenovirus (TsAdV, NC_004453), murine adenovirus A (MAdV-A, AC000012), equine adenovirus 2 (EAdV-2, L80007), human adenovirus 1 (HAdV-1, AC000017), human adenovirus 3 (HAdV-3, DQ086466), human adenovirus 4 (HAdV-4, AY458656), human adenovirus 8 (HAdV-8, AB448769), human adenovirus 12 (HAdV-12, X73487), human adenovirus 40 (HAdV-40, NC_001454), simian adenovirus 21 (SAdV-21, AC000010) and white sturgeon adenovirus (WsAdV, AY082701). Branch lengths are proportional to the number of amino acid substitutions, while vertical distances are for clarity only. The numbers at each node are bootstrap probabilities (expressed as percentages), as determined for 1000 iterations by PAUP version 4.0b.
Table 4.
Nucleotide and amino acid sequence identity between South Polar skua adenovirus 1 and other representative adenoviruses.
| Genus | Virus straina | % identityb | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Polymerase | Penton base | Hexon | Fiber | ||||||
| nt | aa | nt | aa | nt | aa | nt | aa | ||
| Siadenovirus | THEV | 68.3 | 65.6 | 75.4 | 74.7 | 74.9 | 75.4 | 48.0 | 29.1 |
| HEV | 68.3 | 65.6 | 75.4 | 74.7 | 74.9 | 75.4 | 48.0 | 29.1 | |
| TAdV A | 68.3 | 65.6 | 75.4 | 74.7 | 74.9 | 75.4 | 48.0 | 29.1 | |
| RAdV 1 | 73.8 | 73.4 | 79.2 | 82.4 | 77.5 | 85.3 | 57.2 | 43.8 | |
| FrAdV 1 | 59.5 | 51.1 | 66.4 | 64.0 | 66.2 | 69.0 | 47.1 | 29.7 | |
| GTAdV 1 | 68.0 | 77.5 | 72.7 | 71.2 | 61.7 | 55.8 | n.d | n.d | |
| PsAdV | 66.9 | 68.5 | n.d | n.d | 40.7 | 36.4 | n.d | n.d | |
| STAdV 1 | 60.7 | 56.7 | n.d | n.d | n.d | n.d | n.d | n.d | |
| Aviadenovirus | FaAdV | n.d | n.d | 52.5 | 47.3 | 53.3 | 53.0 | n.d | n.d |
| FAdV 1 | 44.5 | 37.9 | 49.0 | 51.0 | 50.6 | 53.1 | 23.7 | 14.8 | |
| Atadenovirus | DAdV 1 | 51.3 | 40.6 | 57.2 | 54.4 | 57.2 | 53.2 | 30.6 | 17.0 |
| SnAdV 1 | 47.4 | 37.4 | 54.7 | 54.3 | 52.8 | 53.8 | 29.2 | 13.4 | |
| OAdV 7 | 54.6 | 40.9 | 60.1 | 53.5 | 60.5 | 54.4 | 30.8 | 15.3 | |
| Mastadenovirus | BAdV 3 | 48.4 | 41.0 | 50.6 | 50.9 | 50.3 | 51.2 | 29.1 | 16.0 |
| CAdV 2 | 47.9 | 40.3 | 49.6 | 48.2 | 52.1 | 52.2 | 28.0 | 16.7 | |
| MAdV A | 49.6 | 39.7 | 45.5 | 47.7 | 52.5 | 50.2 | 29.2 | 14.8 | |
| PAdV A | 41.8 | 36.1 | 50.7 | 48.5 | 47.2 | 50.7 | 28.2 | 18.7 | |
| TsAdV | 48.2 | 39.8 | 52.0 | 49.8 | 50.2 | 53.1 | 35.2 | 19.6 | |
| HAdV 1 | 43.9 | 38.1 | 46.9 | 48.9 | 50.8 | 50.9 | 30.9 | 15.9 | |
| HAdV 3 | 45.5 | 39.6 | 50.5 | 51.0 | 52.2 | 50.6 | 33.4 | 17.5 | |
| HAdV 4 | 45.1 | 42.0 | 46.4 | 50.8 | 48.7 | 51.1 | 30.7 | 17.7 | |
| HAdV 8 | 43.9 | 38.9 | 46.9 | 49.9 | 48.9 | 49.8 | 31.9 | 17.3 | |
| HAdV 12 | 47.8 | 40.8 | 53.0 | 49.7 | 52.3 | 50.3 | 30.5 | 16.0 | |
| HAdV 40 | 44.7 | 38.9 | 39.7 | 50.7 | 51.1 | 50.5 | 29.1 | 19.5 | |
| SAdV 21 | 45.4 | 39.5 | 49.8 | 50.3 | 51.6 | 50.7 | 31.5 | 16.5 | |
Virus strains and GenBank numbers; avirulent turkey hemorrhagic enteritis virus (THEV, AY849321), hemorrhagic enteritis virus (HEV, AF074946), turkey adenovirus A (TAdV-A, AC000016), frog adenovirus (FrAdV-1, AF224336), raptor adenovirus 1 (RAdV-1, EU715130), Sulawesi tortoise adenovirus (STAdV-1, EU056826), Great tit adenovirus (GTAdV, FJ849795), Psittacine adenovirus (PsAdV, pol, EU056825; hexon, EU627198), fowl adenovirus (FAdV-1, U46933), falcon adenovirus (FaAdV, AY683541), duck adenovirus 1 (DAdV-1, Y09598), snake adenovirus (SnAdV-1, DQ106414), Ovine adenovirus 7 (OAdV-7, OAU40839), bovine adenovirus 3 (BAdV-3, AF030154), Canine adenovirus 2 (CAdV-2, AC000020), porcine adenovirus A (PAdV-A, NC_005869), tree shrew adenovirus (TsAdV, NC_004453), murine adenovirus A (MAdV-A, AC000012), human adenovirus 1 (HAdV-1, AC000017), human adenovirus 3 (HAdV-3, DQ086466), human adenovirus 4 (HAdV-4, AY458656), human adenovirus 8 (HAdV-8, AB448769), human adenovirus 12 (HAdV-12, X73487), human adenovirus 40 (HAdV-40, NC_001454) and simian adenovirus 21 (SAdV-21, AC000010). Only partial nucleotide sequence of polymerase gene from PsAdV (269-bps) and STAdV (272-bps), and nucleotide sequence of hexon from GTAdV (2703-bps), PsAdV (587-bps) and FaAdV (sss-bps) were available.
nt; nucleotide, aa; amino acid, n.d: not done.
The fiber gene of SPSAdV revealed only 57.2% and 48.0% nucleotide sequence similarity with that of RAdV-1 and THEV, respectively. Compared with other genera, the pol, penton base and hexon genes of SPSAdV shared < 61% nucleotide sequence similarity with that of Mastadenovirus, Atadenovirus and Aviadenovirus. The nucleotide and amino acid sequences of the pol, penton base, hexon and fiber genes showed nearly equi-distant differences between SPSAdV and other siadenoviruses.
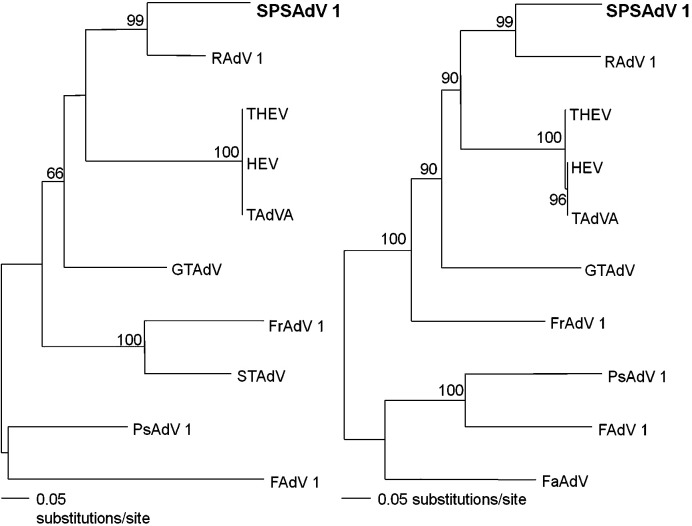
In Fig. 4 , trees were based on the polymerase and hexon genes. Only Siadenovirus and Aviadenovirus sequences were compared. These trees, which also included siadenoviruses from great tit, psittacine and Sulawesi tortoise, showed that SPSAdV was most closely related to RAdV-1.
Fig. 4.
Phylogenetic trees, based on the partial nucleotide sequences of the polymerase (275 bp, left) and hexon (608 bp, right) genes, generated by the neighbor-joining method. Phylogenetic relationships of SPSAdV are shown with other Siadenovirus and Aviadenovirus, including raptor adenovirus 1 (RAdV-1, EU715130), avirulent turkey hemorrhagic enteritis virus (THEV, AY849321), hemorrhagic enteritis virus (HEV, AF074946), turkey adenovirus A (TAdV-A, AC000016), frog adenovirus 1 (FrAdV-1, AF224336), psittacine adenovirus (PsAdV, pol gene: EU056825; hexon gene: EU627198), Sulawesi tortoises adenovirus (STAdV, EU056826), great tit adenovirus (GTAdV, FJ849795), fowl adenovirus 1 (FAdV-1, U46933), falcon adenovirus (FaAdV, AY683541). Branch lengths are proportional to the number of nucleotide substitutions, while vertical distances are for clarity only. The numbers at each node are bootstrap probabilities (expressed as percentages), as determined for 1000 iterations by PAUP version 4.0b.
Discussion
Only a limited number of viruses have hitherto been discovered among animals in the Polar region. Infectious bursal disease virus (IBDV) and poxvirus were detected in penguins (Gauthier-Clere et al., 2002, Stannard et al., 1998) and serum antibodies to influenza A viruses and paramyxoviruses were reported in skua and Adelie penguin in the Ross Sea in Antarctica (Austin and Webster, 1993). In this study, viruses were targeted for discovery in Antarctic birds. Although no evidence of influenzavirus and coronavirus was found, a novel adenovirus was detected by PCR in the South Polar skua, a predatory seabird species whose migratory route includes Antarctica.
Based on genetic and phylogenetic analyses, the newly identified viral sequences from six South Polar skuas could be classified as a novel siadenovirus. Other members of the genus Siadenovirus include THEV (Beach et al., 2009), RAdV-1 (Kovács and Benkö, 2011) and great tit adenovirus (GTAdV) (Kovács et al., 2010), all from avian hosts, as well as frog adenovirus 1 (FrAdV-1) (Davison et al., 2000), originating from an amphibian host. At first, we assumed that SPSAdV would belong to the Aviadenovirus genus because the South Polar skua is an Antarctic bird. However, phylogenetic analysis revealed that SPSAdV was similar to RAdV-1 and THEV (Pitcovski et al., 1998). Comparison between SPSAdV and its closest relatives (RAdV-1 and THEV) showed 21–43% and 25–52% nucleotide dissimilarity at the pol, penton base, hexon and fiber genes, and 15–56% and 25–71% amino acid difference, respectively. Also, the nucleotide sequences of the pol, penton base, hexon and fiber genes of SPSAdV, compared with FrAdV-1, showed 34–53% dissimilarity. Interestingly, although birds serve as host species of aviadenoviruses (Jiang et al., 1999, Oaks et al., 2005, Schrenzel et al., 2005), Aviadenovirus encodes more distinct proteins than Siadenovirus (Benkö et al., 2000). The G + C content of the SPSAdV (34.2%) is similar with that of the other three siadenoviruses (RAdV-1: 38.5%, TAdV-3: 34.9%, FrAdV-1: 37.9%). The pVII gene of SPSAdV also shows significantly higher G + C content (46.9%). The G + C content does not vary across the genome in a systematic fashion, and this may suggest that a recombination event between disparate viruses did not occur.
Designation of a novel siadenovirus species is predicated on more than 10% sequence dissimilarity at the nucleotide and amino acid levels and a previously unrecognized host species (Benkö et al., 2000, Benkö et al., 2005). Based on these criteria, we conclude that SPSAdV represents a novel adenovirus species in the genus Siadenovirus. Recently, the entire genome of RAdV-1 was obtained by PCR without virus isolation (Kovács and Benkö, 2011). Thus, apart from THEV, RAdV-1 and FrAdV-1, this is only the fourth complete viral genome sequence in the genus Siadenovirus. Partial siadenovirus genomes have also been reported from the great tit (Kovács et al., 2010), budgerigar (Katoh et al., 2009), psittacine (Wellehan et al., 2009) and Sulawesi tortoise (Rivera et al., 2009). Siadenovirus from different avian, reptilian and amphibian host species share the same genome organization (Kovács and Benkö, 2009), suggesting that the evolutionary history may have involved host-switching events (Davison et al., 2003, Kovács and Benkö, 2009).
Other than the genetic and phylogenetic features, an important consideration is the clinical signs of adenovirus infection. It is well known that THEV infection is occasionally lethal and is characterized by depression, diarrhea, splenomegaly (Beach et al., 2009). Because of the close phylogenetic relationship between THEV and SPSAdV, we speculate that certain disease manifestations may be shared. That six of nine dead South Polar skuas had evidence of SPSAdV genomic sequences, as determined by PCR in one or more organs (heart, lung, liver, kidney, intestine and/or trachea), suggests systemic or disseminated infection, presumably with viremia and clinically significant disease outcome. SPSAdV infection in the South Polar skua may indicate acquisition and spread of infection as a result of stress from migration. Future studies are warranted to ascertain the biology, epizootiology and pathogenic potential of this newfound polar-region siadenovirus.
Materials and methods
Samples
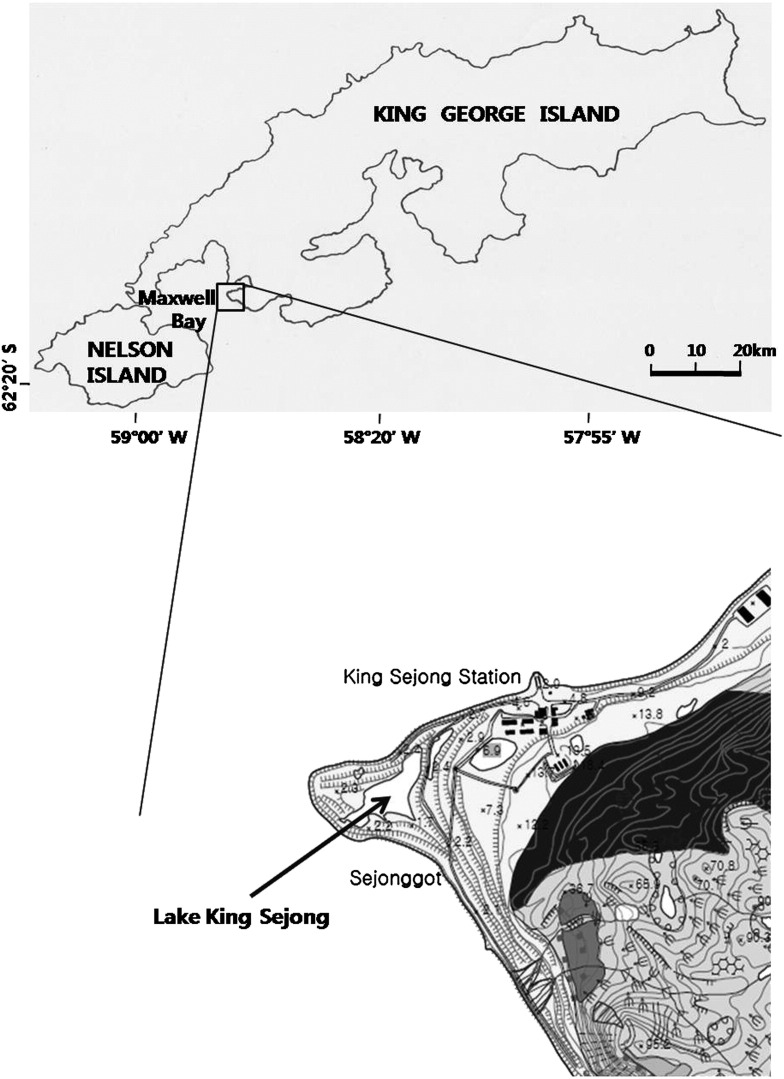
Frozen carcasses of nine South Polar skuas (SPS T01-T09), without readily discernable signs of disease, were collected in Lake King Sejong near King Sejong station (latitude 62° 13′ S and longitude 58° 47′ W) in Antarctica (Fig. 1), when ambient temperatures ranged from − 5.6 °C to 2.1 °C. Tissue samples from various organs (heart, trachea, lung, esophagus, intestine, liver, kidney) were obtained using separate sterile instruments from each bird and stored at − 70 °C until used. Autopsy was conducted in a BSL2 laboratory.
Fig. 1.
Collection site of South Polar skua carcasses. Lake King Sejong is located southeast of King Sejong station, on King George Island, in Antarctica.
PCR and DNA sequencing
Total DNA was extracted from blood and tissue samples using the High Pure PCR Template preparation kit (Roche, Indianapolis, IN), according to the manufacturer's instructions. First and nested PCR were performed in a 50-μL reaction volume containing 1 μL of 10 mM dNTP, 2 μL (10 pM) of each primer, 1 unit of Super-Therm Taq polymerase (JMR Holdings, London, UK) and 2.5 μL (400 ng) of template. Primers used for PCR amplification and sequencing are provided in Table 1.
Table 1.
Oligonucleotide primers for full genome amplification of South Polar skua adenovirus.
| Gene | Primer | Nucleotide sequence (5′–3′) | Polarity |
|---|---|---|---|
| ITR | Adv-ITR_EcoR I | 5′-GAA TTC CA ATC AAT ATA TAT ACC-3′ | +/− |
| IVa2 | Adv-IVa2R2926 | 5′-ACC TAG ATA TCA ACA ATG A-3′ | − |
| Polymerase | Adv-polR VI | 5′-CTG TCK GTR TCD CCA TA-3′ | + |
| Adv-PolFouter_ku | 5′-TCM GAG GBG GAC GAT GYT ACC C-3′ | − | |
| Adv-Pol707R | 5′-GAT ACC CAA CTC AAC TAG CA-3′ | − | |
| Adv-PolF4052 | 5′-TCG TCA GAG TAT AGA TAG TC-3′ | + | |
| Adv-PolR3452 | 5′-TAC AGG AAT TCG AAG AT-3′ | − | |
| Adv-PolF3992 | 5′-AGA CTG TCA GTA TCA-3′ | + | |
| pTP | Adv-ptp6783R | 5′-ACT AAG AGC ACC AAG ATG A-3′ | − |
| Adv-ptp68F | 5′-TAC TTG TGG TAA CTA GA-3′ | + | |
| 52K | Adv-52K8696R | 5′-TCT CCA TTT GCT CAG TA-3′ | − |
| Adv-52K9279F | 5′-TAG GTG TAC AAA CTA GA-3′ | + | |
| Penton base | Adv-Pen11161R | 5′-GAA TGA TCT TTA TCC TGA T-3′ | − |
| Adv-Pen241F | 5′-GAT AAC AAG GCV ADT GAT AT-3′ | + | |
| Adv-Pen597R | 5′-TCA ATA ADC TCA TT-3′ | − | |
| Adv-Pen869F | 5′-ATT RAR TAT GAT GA-3′ | + | |
| Hexon | Adv-Hex13700R | 5′-AAT CTA CGA GAT TCT CTG A-3′ | − |
| Adv-Hex16F | 5′-ATG GAY ATW TCA AAT GCT AC-3′ | + | |
| Adv-Hex409R | 5′-ATT GAG CTG ACC TTG GAG C-3′ | − | |
| Adv-Hex1159F | 5′-TGG AAY CAA GCT GTW GA-3′ | + | |
| DBP | Adv-DBP17732F | 5′-ATG GAA GCA TCT GA-3′ | + |
| 100K | Adv-100K19311R | 5′-ATG CTG TCA ACC AT-3′ | − |
| Adv-100K19813F | 5′-AGC TTT ACA CAA TGA-3′ | + | |
| Adv-100K19278F | 5′-TGA ATG ATG GTG AAG A-3′ | + | |
| Adv-100K19995R | 5′-TTC TCA GGA TAA TCC A-3′ | − | |
| E3 | Adv-E3R21725 | 5′-ACA CAA GCT GAA GCA-3′ | − |
| Adv-E3F21275 | 5′-TAC AGG AGG AGC TCT GT-3′ | + | |
| Fiber | Adv-Fiber23068R | 5′-ATC CAA GAC CAT TAC CAA-3′ | − |
| Adv-Fiber23030F | 5′-GTG GTA TGC TTA GTT TGA-3′ | + |
Initially, adenovirus sequences available in GenBank were aligned using Clustal W, MegAlign program. Regions exhibiting high homology were then selected for designing oligonucleotide primers. For amplification of the full genome, specific primers were designed based on newly acquired sequences. Initial denaturation at 95 °C for 5 min was followed by six cycles each of denaturation at 94 °C for 30 s, annealing at 37 °C for 30 s and elongation at 72 °C for 1 min, then 32 cycles of denaturation at 94 °C for 30 s, annealing at 42 °C for 30 s and elongation at 72 °C for 1 min and finally 72 °C for 5 min in a Peltier PTC-200 thermal cycler (MJ Research, Inc., Watertown, MA). Elongation time was altered between 1 and 3 min depending on the expected product size. PCR products were separated by electrophoresis in 1% agarose gels containing ethidium bromide. The right and left ends of the genome were determined by RACE PCR kit (Takara, Shiga, Japan) and amplified by PCR using enzyme-tagged primer in Table 1 (Kovács and Benkö, 2011).
Amplicons were purified by using a PCR Purification Kit (QIAGEN, Chatsworth, CA) and were sequenced with the Big-Dye® Terminator kit version 3.1 (Applied Biosystems, Foster City, CA) and ABI 3730 automated sequencer (Applied Biosystems) after cloning into the pSTBlue-I vector (Novagen, San Diego, CA).
Phylogenetic analysis
The identity of the sequences was searched by Blast (Altschul et al., 1990). Sequences were edited with EditSeq programs in the Lasergene 6 (DNASTAR) (www.dnastar.com) and aligned using Clustal W (Thompson et al., 1994). Phylogenetic trees were constructed, using maximum-likelihood (ML) and neighbor-joining (NJ) algorithms implemented, rooted at the midpoint, in PAUP (Swofford, 2003) based on the full length of amino acid sequences of polymerase, penton base and hexon. The NJ trees using the partial nucleotide sequences of polymerase and hexon were performed for the analysis of partially characterized siadenoviruses including viruses from parrots, great tit and Sulawest tortoises. An initial ML tree estimation was performed by Modeltest 3.7 (Posada and Crandall, 1998). Topologies were evaluated by bootstrap analysis of 1000 NJ and 100 ML replicates. The genetic distances were computed by the PAUP program.
Acknowledgments
We thank Dr. Hae Ji Kang for phylogenetic analysis and Dr. Richard Yanagihara for editorial assistance. This research was supported in part by a Core Research Support for Senior Researchers, National Research Foundation of Korea (2010-002-7564), KOPRI (grant number PE11030), and Institute of Biomedical Science & Food Safety, Korea University.
References
- Aggarwal N., Mittal S.K. Sequence analysis of porcine adenovirus type 3 E1 region, pIX and pIVa2 genes, and two novel open reading frames. Intervirology. 2000;43(1):6–12. doi: 10.1159/000025016. [DOI] [PubMed] [Google Scholar]
- Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. Basic local alignment search tool. J. Mol. Biol. 1990;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Austin F.J., Webster R.G. Evidence of ortho- and paramyxoviruses in fauna from Antarctica. J. Wildl. Dis. 1993;29(4):568–571. doi: 10.7589/0090-3558-29.4.568. [DOI] [PubMed] [Google Scholar]
- Beach N.M., Duncan R.B., Larsen C.T., Meng X.J., Sriranganathan N., Pierson F.W. Comparison of 12 turkey hemorrhagic enteritis virus isolates allows prediction of genetic factors affecting virulence. J. Gen. Virol. 2009;90(Pt 8):1978–1985. doi: 10.1099/vir.0.010090-0. [DOI] [PubMed] [Google Scholar]
- Benkö M., Harrach B., Russell W.C. Family Adenoviridae. In: Van Regenmortel M.H.V., Fauquet C.M., Bishop D.H.L., Carstens E., Estes M., Lemon S., Maniloff J., Mayo M.A., McGeoch D., Pringle C., Wickner R., editors. Virus Taxonomy. VIIth Report of the International Committee on Taxonomy of Viruses Academic Press; New York: 2000. [Google Scholar]
- Benkö M., Harrach B., Both G.W., Russell W.C., Adair B.M., Ádám É., de Jong J.C., Hess M., Johnson M., Kajon A., Kidd A.H., Lehmkuhl H.D., Li Q.G., Mautner V., Pring-Akerblom P., Wadell G. Adenoviridae. In: Fauquet C.M., Mayo M.A., Maniloff J., Desselberger U., Ball L.A., editors. Virus Taxonomy, VIIIth Report of the International Committee on Taxonomy of Viruses. Elsevier, Academic Press; London: 2005. [Google Scholar]
- Dan A., Ruzsics Z., Russell W.C., Benkö M., Harrach B. Analysis of the hexon gene sequence of bovine adenovirus type 4 provides further support for a new adenovirus genus (Atadenovirus) J. Gen. Virol. 1998;79(Pt 6):1453–1460. doi: 10.1099/0022-1317-79-6-1453. [DOI] [PubMed] [Google Scholar]
- Davison A.J., Harrach B. Siadenovirus. In: Tidona C.A., Darai G., editors. The Springer Index of Viruses. Springer-Verlag; Berlin: 2002. [Google Scholar]
- Davison A.J., Wright K.M., Harrach B. DNA sequence of frog adenovirus. J. Gen. Virol. 2000;81(Pt 10):2431–2439. doi: 10.1099/0022-1317-81-10-2431. [DOI] [PubMed] [Google Scholar]
- Davison A.J., Benkö M., Harrach B. Genetic content and evolution of adenoviruses. J. Gen. Virol. 2003;84(Pt 11):2895–2908. doi: 10.1099/vir.0.19497-0. [DOI] [PubMed] [Google Scholar]
- Farkas S.L., Harrach B., Benkö M. Completion of the genome analysis of snake adenovirus type 1, a representative of the reptilian lineage within the novel genus Atadenovirus. Virus Res. 2008;132(1–2):132–139. doi: 10.1016/j.virusres.2007.11.009. [DOI] [PubMed] [Google Scholar]
- Gauthier-Clere M., Eterradossi N., Toquin D., Guittet M., Kuntz G., Maho Y.L. Serological survey of the king penguin, Aptenodytes patagonicus, in Crozet Archipelago for antibodies to infectious bursal disease, influenza A and Newcastle disease viruses. Polar Biol. 2002;25:316–319. [Google Scholar]
- Goldstein T., Colegrove K.M., Hanson M., Gulland F.M. Isolation of a novel adenovirus from California sea lions Zalophus californianus. Dis. Aquat. Organ. 2011;94(3):243–248. doi: 10.3354/dao02321. [DOI] [PubMed] [Google Scholar]
- Houng H.S., Clavio S., Graham K., Kuschner R., Sun W., Russell K.L., Binn L.N. Emergence of a new human adenovirus type 4 (Ad4) genotype: identification of a novel inverted terminal repeated (ITR) sequence from majority of Ad4 isolates from US military recruits. J. Clin. Virol. 2006;35(4):381–387. doi: 10.1016/j.jcv.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Jiang P., Ojkic D., Tuboly T., Huber P., Nagy E. Application of the polymerase chain reaction to detect fowl adenoviruses. Can. J. Vet. Res. 1999;63(2):124–128. [PMC free article] [PubMed] [Google Scholar]
- Jucker M.T., McQuiston J.R., van den Hurk J.V., Boyle S.M., Pierson F.W. Characterization of the haemorrhagic enteritis virus genome and the sequence of the putative penton base and core protein genes. J. Gen. Virol. 1996;77(Pt 3):469–479. doi: 10.1099/0022-1317-77-3-469. [DOI] [PubMed] [Google Scholar]
- Katoh H., Ohya K., Kubo M., Murata K., Yanai T., Fukushi H. A novel budgerigar-adenovirus belonging to group II avian adenovirus of Siadenovirus. Virus Res. 2009;144(1–2):294–297. doi: 10.1016/j.virusres.2009.04.012. [DOI] [PubMed] [Google Scholar]
- Klempa B., Kruger D.H., Auste B., Stanko M., Krawczyk A., Nickel K.F., Uberla K., Stang A. A novel cardiotropic murine adenovirus representing a distinct species of mastadenoviruses. J. Virol. 2009;83(11):5749–5759. doi: 10.1128/JVI.02281-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovács E.R., Benkö M. Confirmation of a novel siadenovirus species detected in raptors: partial sequence and phylogenetic analysis. Virus Res. 2009;140(1–2):64–70. doi: 10.1016/j.virusres.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Kovács E.R., Benkö M. Complete sequence of raptor adenovirus 1 confirms the characteristic genome organization of siadenoviruses. Infect. Genet. Evol. 2011;11(5):1058–1065. doi: 10.1016/j.meegid.2011.03.021. [DOI] [PubMed] [Google Scholar]
- Kovács G.M., Davison A.J., Zakhartchouk A.N., Harrach B. Analysis of the first complete genome sequence of an Old World monkey adenovirus reveals a lineage distinct from the six human adenovirus species. J. Gen. Virol. 2004;85(Pt 10):2799–2807. doi: 10.1099/vir.0.80225-0. [DOI] [PubMed] [Google Scholar]
- Kovács E.R., Janoska M., Dan A., Harrach B., Benkö M. Recognition and partial genome characterization by non-specific DNA amplification and PCR of a new siadenovirus species in a sample originating from Parus major, a great tit. J. Virol. Methods. 2010;163(2):262–268. doi: 10.1016/j.jviromet.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Lehmkuhl H.D., Hobbs L.A. Serologic and hexon phylogenetic analysis of ruminant adenoviruses. Arch. Virol. 2008;153(5):891–897. doi: 10.1007/s00705-008-0063-4. [DOI] [PubMed] [Google Scholar]
- Li Y., Ge X., Zhang H., Zhou P., Zhu Y., Zhang Y., Yuan J., Wang L.F., Shi Z. Host range, prevalence, and genetic diversity of adenoviruses in bats. J. Virol. 2010;84(8):3889–3897. doi: 10.1128/JVI.02497-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mase M., Mitake H., Inoue T., Imada T. Identification of group I –III avian adenovirus by PCR coupled with direct sequencing of the hexon gene. J. Vet. Med. Sci. 2009;71(9):1239–1242. doi: 10.1292/jvms.71.1239. [DOI] [PubMed] [Google Scholar]
- Morrison M.D., Onions D.E., Nicolson L. Complete DNA sequence of canine adenovirus type 1. J. Gen. Virol. 1997;78(Pt 4):873–878. doi: 10.1099/0022-1317-78-4-873. [DOI] [PubMed] [Google Scholar]
- Oaks J.L., Schrenzel M., Rideout B., Sandfort C. Isolation and epidemiology of falcon adenovirus. J. Clin. Microbiol. 2005;43(7):3414–3420. doi: 10.1128/JCM.43.7.3414-3420.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcovski J., Mualem M., Rei-Koren Z., Krispel S., Shmueli E., Peretz Y., Gutter B., Gallili G.E., Michael A., Goldberg D. The complete DNA sequence and genome organization of the avian adenovirus, hemorrhagic enteritis virus. Virology. 1998;249(2):307–315. doi: 10.1006/viro.1998.9336. [DOI] [PubMed] [Google Scholar]
- Posada D., Crandall K.A. MODELTEST: testing the model of DNA substitution. Bioinformatics. 1998;14(9):817–818. doi: 10.1093/bioinformatics/14.9.817. [DOI] [PubMed] [Google Scholar]
- Rivera S., Wellehan J.F., Jr., McManamon R., Innis C.J., Garner M.M., Raphael B.L., Gregory C.R., Latimer K.S., Rodriguez C.E., Diaz-Figueroa O., Marlar A.B., Nyaoke A., Gates A.E., Gilbert K., Childress A.L., Risatti G.R., Frasca S., Jr. Systemic adenovirus infection in Sulawesi tortoises (Indotestudo forsteni) caused by a novel siadenovirus. J. Vet. Diagn. Invest. 2009;21(4):415–426. doi: 10.1177/104063870902100402. [DOI] [PubMed] [Google Scholar]
- Russell W.C. Adenoviruses: update on structure and function. J. Gen. Virol. 2009;90(Pt 1):1–20. doi: 10.1099/vir.0.003087-0. [DOI] [PubMed] [Google Scholar]
- Rusvai M., Harrach B., Banrevi A., Evans P.S., Benkö M. Identification and sequence analysis of the core protein genes of bovine adenovirus 2. Virus Res. 2000;70(1–2):25–30. doi: 10.1016/s0168-1702(00)00201-x. [DOI] [PubMed] [Google Scholar]
- Rux J.J., Burnett R.M. Adenovirus structure. Hum. Gene Ther. 2004;15(12):1167–1176. doi: 10.1089/hum.2004.15.1167. [DOI] [PubMed] [Google Scholar]
- Schrenzel M., Oaks J.L., Rotstein D., Maalouf G., Snook E., Sandfort C., Rideout B. Characterization of a new species of adenovirus in falcons. J. Clin. Microbiol. 2005;43(7):3402–3413. doi: 10.1128/JCM.43.7.3402-3413.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stannard L.M., Marais D., Kow D., Dumbell K.R. Evidence for incomplete replication of a penguin poxvirus in cells of mammalian origin. J. Gen. Virol. 1998;79(Pt 7):1637–1646. doi: 10.1099/0022-1317-79-7-1637. [DOI] [PubMed] [Google Scholar]
- Swofford D. 2003. PAUP*: Phylogenetic Analysis Using Parsimony (*and Other Methods), Version 4 Sinauer Associates, Sunderland, Massachusetts. [Google Scholar]
- Thompson J.D., Higgins D.G., Gibson T.J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22(22):4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson D., Meers J., Harrach B. Molecular confirmation of an adenovirus in brushtail possums (Trichosurus vulpecula) Virus Res. 2002;83(1–2):189–195. doi: 10.1016/S0168-1702(01)00437-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber J.M. Synthesis and assay of recombinant adenovirus protease. Methods Mol. Med. 2007;131:251–255. doi: 10.1007/978-1-59745-277-9_18. [DOI] [PubMed] [Google Scholar]
- Wellehan J.F., Johnson A.J., Harrach B., Benkö M., Pessier A.P., Johnson C.M., Garner M.M., Childress A., Jacobson E.R. Detection and analysis of six lizard adenoviruses by consensus primer PCR provides further evidence of a reptilian origin for the atadenoviruses. J. Virol. 2004;78(23):13366–13369. doi: 10.1128/JVI.78.23.13366-13369.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellehan J.F., Jr., Greenacre C.B., Fleming G.J., Stetter M.D., Childress A.L., Terrell S.P. Siadenovirus infection in two psittacine bird species. Avian Pathol. 2009;38(5):413–417. doi: 10.1080/03079450903183660. [DOI] [PubMed] [Google Scholar]
- Yogui G.T., Sericano J.L. Levels and pattern of polybrominated diphenyl ethers in eggs of Antarctic seabirds: endemic versus migratory species. Environ. Pollut. 2009;157(3):975–980. doi: 10.1016/j.envpol.2008.10.016. [DOI] [PubMed] [Google Scholar]