Abstract
Emerging and well-known viral diseases remain one the most important global public health threats. A better understanding of their pathogenesis and mechanisms of transmission requires animal models that accurately reproduce these aspects of the disease. Here we review the role of ferrets as an animal model for the pathogenesis of different respiratory viruses with an emphasis on influenza and paramyxoviruses. We will describe the anatomic and physiologic characteristics that contribute to the natural susceptibility of ferrets to these viruses, and provide an overview of the approaches available to analyze their immune responses. Recent insights gained using this model will be highlighted, including the development of new prophylactic and therapeutic approaches. To provide decision criteria for the use of this animal model, its strengths and limitations will be discussed.
Keywords: Ferret, Animal model, Paramyxoviruses, Influenza viruses, Pathogenesis studies, Vaccine and drug safety and efficacy assessment, Immune response evaluation
Highlights
- •
Ferrets as models for respiratory virus pathogenesis.
- •
Ferrets as models for vaccine and drug efficacy assessment.
- •
Immunological tools for ferrets.
- •
Housing and handling of ferrets.
Ferrets as experimental animals
Domestic ferrets (Mustela putorius furo) are small carnivores belonging to the Mustelidae family. Their closest wild relatives are wild European ferrets (M. putorius), European polecats (M. furo) and the steppe polecat (M. eversmannii), and interbreeding among these species produces fertile offspring, illustrating the close relationship (Lewington, 2007). Due to their relatively small size and their similarity to humans with respect to aspects of their anatomy, physiology, and metabolism, they are increasingly considered an alternative to larger animal models such as dogs and non-human primates (Clingerman et al., 1991). Ferrets are available from different commercial breeders, some of which are even offering specific pathogen free (SPF) animals. Seronegative animals, while of particular interest for the infectious disease community, are difficult to maintain due to the natural susceptibility of ferrets for human respiratory viruses. Breeders offer pre-testing of animals prior to purchase and use HEPA-filtered transport cages to minimize risk of exposure during shipping, but the serostatus of all animals has to be verified after arrival. In addition, personal protective equipment such as N95 masks or powered air purifying respirators (PAPRs) should be worn for all interactions with the animals to prevent accidental exposure by infected staff.
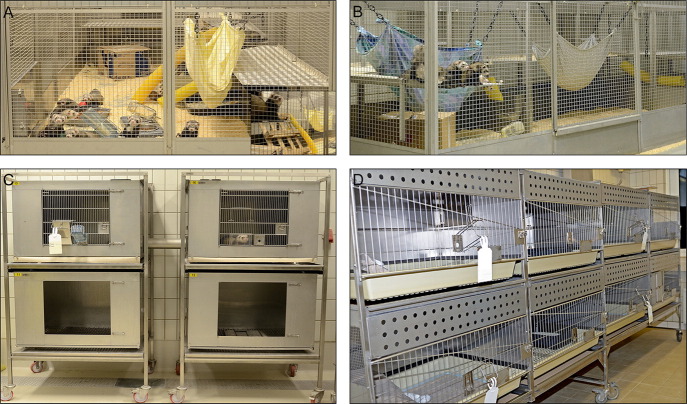
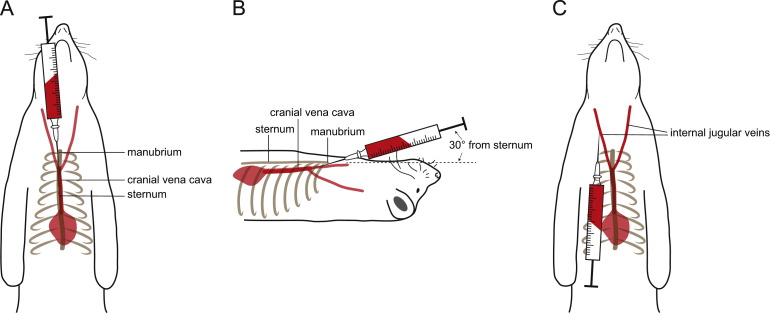
The animals are social and should be housed in same sex groups whenever possible ( Fig. 1A and B). Many of the standard rabbit caging systems can be used to house ferrets during experiments (Fig. 1C and D). The spacing of the grid walls and food receptacles may have to be adapted to prevent escape, and hiding places such as tubing or tunnel, hammocks, or nesting boxes have to be provided (Ball, 2002). Because of their value for pathogenesis studies with viruses requiring high containment, different individually ventilated cage (IVC) systems have been also been developed. An adult female ferret weighs 700–1000 g, while males weigh 1400–2000 g, thus allowing repeat blood sampling at volumes sufficient for immunological analyses over the course of an infection (Bixler and Ellis, 2004). While small blood samples can be obtained from the tail or leg veins, larger volumes required for the isolation of peripheral blood mononuclear cells are best collected from the anterior vena cava ( Fig. 2A and B) or the internal jugular vein (Fig. 2C). The sexual weight dimorphism together with the requirement for inducing ovulation in female ferrets in estrus has led many researchers to preferentially use males (Fox, 1998, Sherrill and Gorham, 1985).
Fig. 1.
Examples of different cage types for ferret housing. Longterm free-range housing in same sex groups. Environmental enrichment like hammocks, tubes, and additional levels for climbing are provided (A, B). During ongoing experiments the animals are housed in pairs in different cage types connected by tubes or openings in separating walls (C, D).
Fig. 2.
Large volume blood collection sites. The anterior vena cava (A, B) and the internal jugular vein (C) allow collection of larger blood volumes.
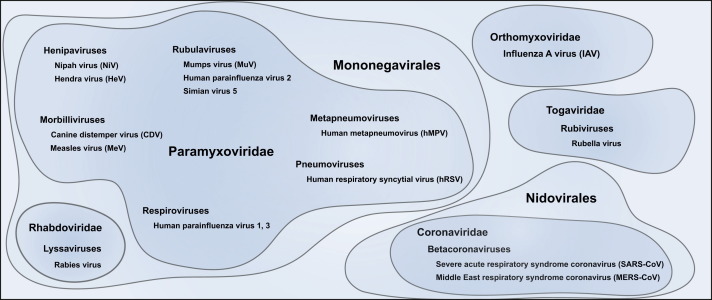
Following the fortuitous discovery of the natural susceptibility of ferrets to human influenza viruses in the 1930s (Smith et al., 1933), they were found to reproduce the human course of disease of a number of human respiratory viruses including respiratory syncytial virus (RSV), parainfluenzaviruses (PIV), and severe acute respiratory syndrome (SARS)-coronavirus ( Fig. 3). In addition to the presence of the respective receptors, the fact that anatomic proportions of the ferret upper and lower respiratory tracts, the density of submucosal glands in the bronchial wall and the number of generations of terminal bronchioles are all reproduce the situation in the human respiratory tract likely contributes to this effect (Johnson-Delaney and Orosz, 2011, Robinson et al., 1986). With technical developments enabling infection via the ocular route or by aerosol, the impact of the inoculation route and volume on pathogenesis have been increasingly recognized (Belser et al., 2014, Belser et al., 2012, Bodewes et al., 2013, Kreijtz et al., 2013, Moore et al., 2014). For most studies however, the animals are inoculated intranasally with volumes ranging from 0.1 to 1 ml.
Fig. 3.
Viruses investigated in ferrets. Viruses from different families discussed in this review that have been studied in ferrets.
Influenza virus
In the context of infectious disease research, ferrets are most widely known as model for the study of influenza viruses, which belong to the Orthomyxoviridae family (Palese and Shaw, 2007). Influenza A, and to a lesser extent influenza B, viruses cause annual epidemics with an estimated 250,000–500,000 deaths worldwide. While humans are the only natural reservoir for influenza B, influenza A infects a broad range of avian and mammalian species with aquatic birds acting as principal reservoir. Among livestock, infections in poultry and pigs have the greatest economic impact, but horses, and more recently dogs, can also be affected. Avian and swine influenza A viruses are of particular public health relevance because their close contact with humans provides multiple opportunities for interspecies adaptation and transmission (Palese and Shaw, 2007).
Influenza A viruses are grouped into different subtypes according to the antigenicity of their surface glycoproteins, which are the principal targets of the neutralizing antibody response (Gerhard, 2001, Palese and Shaw, 2007). So far, 18 hemagglutinin (HA, H1–H18) and 11 neuraminidase (NA, N1–N11) subtypes have been identified (Tong et al., 2012, Tong et al., 2013). Introduction of a new influenza A subtype into the human population can result in a pandemic with increased morbidity and mortality (Belser et al., 2009, Johnson and Mueller, 2002). Avian influenza viruses are further classified in low pathogenic (LPAIV) and highly pathogenic avian influenza viruses (HPAIV), according to their HA cleavage site: LPAIV HAs carry a monobasic cleavage site, which can only be activated by proteases present in the avian respiratory tract, while HPAIV HAs are activated by subtilisin-like proteases present in every cell due to their polybasic cleavage site (Bertram et al., 2010).
Ferrets became the animal model of choice for influenza research when the first isolation of an influenza virus succeeded in this species (Smith et al., 1933), and they have made remarkable contributions to our understanding of influenza biology ever since. In addition to being naturally susceptible to human influenza A strains, the clinical course of disease in ferrets reproduces key aspects of human disease including fever, lethargy and signs of upper and sometimes lower respiratory infection (Bouvier and Lowen, 2010, Smith and Sweet, 1988). The similar influenza receptor distribution in the human and ferret respiratory system likely represents an important contributing factor: in the upper respiratory tract of both species, there is a high density of alpha 2,6-linked sialic acids (SAα2,6), while SAα2,6 as well as alpha 2,3-linked sialic acids (SAα2,3) are present in the lower respiratory tract (Shinya et al., 2006, van Riel et al., 2007). As seen in adult human patients with uncomplicated influenza, infection of ferrets with seasonal H1N1 and H3N2 strains, which preferentially bind to SAα2,6, results in a mild to moderate disease characterized by extensive infection of the upper respiratory tract with limited spread to the lung (Huang et al., 2011, van den Brand et al., 2012). In contrast, highly pathogenic influenza viruses, which have a higher affinity to SAα2,3, are always associated with severe pneumonia and widespread infection throughout the lung (Belser et al., 2009, Kumlin et al., 2008, Nicholls et al., 2008, Shinya et al., 2006, van Riel et al., 2007), and in the case of certain strains also lead to neurologic and gastrointestinal involvement (Gambotto et al., 2008). The assessment of the reconstructed pandemic H1N1 1918 virus in ferrets not only demonstrated the high virulence of this historic virus compared to a seasonal human H1N1 strain (Taubenberger et al., 1997, Tumpey et al., 2005), but also revealed that two amino acid changes in the HA protein were sufficient to switch the receptor specificity from SAα2,6 to SAα2,3 and abolish respiratory droplet transmission (Tumpey et al., 2007), illustrating the important role of receptor specificity in influenza pathogenesis.
The similarity in influenza susceptibility between humans and ferrets is increasingly exploited to gain insights into the transmissibility and virulence of new isolates. Human-adapted seasonal influenza viruses spread through direct or indirect contact or respiratory droplets (Brankston et al., 2007), while infections with avian viruses are usually associated with close contact to infected poultry and are rarely transmitted. Transmissibility assessments of different HPAIs in ferrets have confirmed the poor transmissibility observed in the field (Maines et al., 2006). However, there is great concern that one of these viruses acquires the capacity for aerosol spread and causes a pandemic (Matrosovich et al., 2000, Stevens et al., 2008). Characterization of transmission determinants using genetically modified H5N1 viruses revealed that only five amino acid changes in the HA, PB1, and PB2 proteins were sufficient to enable efficient respiratory droplet transmission between ferrets (Herfst et al., 2012, Imai et al., 2012, Linster et al., 2014). Most of these mutations had already been described to play an important role in influenza virulence in ferrets and mice by either affecting replication efficiency or altering virus tropism (Chen et al., 2007, Hatta et al., 2001, Maines et al., 2011, Shinya et al., 2004). In contrast to HPAI H5N1, the recently emerged human H7N9 isolates display a natural affinity for both SAα2,6 and SAα2,3 receptors. Even though they replicate efficiently in the upper and lower respiratory tract of humans and ferrets, transmission studies have so far yielded contradictory results ranging from reports of efficient airborne transmission over reduced aerosol transmission compared to seasonal and pandemic H1N1 strains to contact transmission only (Belser et al., 2013, Richard et al., 2013).
The value of the ferret model to rapidly gauge the virulence of a newly emerging virus first became apparent during the H1N1 2009 pandemic. Evaluation of early isolates indicated an increased morbidity and higher pathogenicity compared to seasonal H1N1 strains but similar transmission efficiency (Maines et al., 2009, Munster et al., 2009), thereby providing valuable information to public health officials. A large body of work investigating the pathogenesis and transmissibility of HPAI H5N1, H7N1, and H9N1 strains isolated from human cases or poultry provides a framework for the pandemic risk assessment of new viruses emerging from the animal reservoir (Kimble et al., 2014, Lipatov et al., 2009, Maines et al., 2006, Sutton et al., 2014, Yen et al., 2007). Recently, avian H3N8 influenza viruses isolated from seals were found to have naturally acquired mutations known to increase transmissibility, demonstrated increased affinity for mammalian SAα2,6 receptors, and were transmitted via respiratory droplets between ferrets (Karlsson et al., 2014), suggesting a pandemic potential for these viruses. This year, a novel highly pathogenic H5N8 virus was isolated from outbreaks in poultry first in Korea and China, and more recently also in Europe and Canada (Fan et al., 2014, Ku et al., 2014, Wu et al., 2014). The virus causes moderate to severe disease in ferrets, but so far no avian-to-human transmission has been observed (Kim et al., 2014). This continued emergence of new influenza strains in birds and mammals not only requires active surveillance but also the need for systematic characterization of their pandemic potential including pathogenesis studies in ferrets.
Despite the limited availability of ferret-specific immunological reagents, investigation of innate host responses based on mRNA expression levels, either by quantitative real-time RT-PCR or using a canine microarray, revealed profiles similar to those seen in patients, thereby illustrating the value of this animal model not only for virulence assessment but also the characterization of host responses (Cameron et al., 2008, Kang et al., 2011, Maines et al., 2012, Meunier et al., 2012). While seasonal influenza strains are generally associated with a rapid rise and fall of type I and II interferons and TNFα during the first days after infection (Rowe et al., 2010, Svitek et al., 2008), highly pathogenic H5N1 strains lead to a more sustained expression of interferon response genes and higher expression levels of cytokines and inflammatory mediators in the lungs (Cameron et al., 2008). Since such a hypercytokinemia or cytokine storm is also observed in H5N1 patients (de Jong et al., 2006, Peiris et al., 2009, To et al., 2001) a more detailed characterization of the underlying pathomechanism in ferrets, using newly developed tools and assays and including more detailed kinetics of lymphocyte subsets, hematological parameters and serum chemistry profiles (Belser et al., 2011), may yield innovative therapeutic approaches.
The ferret model also remains the gold standard for efficacy assessments of new vaccine strategies and therapeutic approaches. These studies are usually performed using seronegative animals to eliminate additional confounding factors, even though this does not reflect the epidemiological situation in the human population, where most adults have been exposed to at least one, but likely several, strains of the circulating subtypes (Bodewes et al., 2011, Fonville et al., 2014, Hoschler et al., 2013). At this time, inactivated influenza vaccines and live-attenuated influenza vaccines (LAIV) are licensed in most countries (Lambert and Fauci, 2010). While LAIV induce a stronger immune response in naïve individuals, inactivated vaccines are more immunogenic in the presence of pre-existing immunity. In the context of the annual vaccination campaigns, LAIV are thus primarily given to children and young adults (Rodgers et al., 2015). The potential of LAIV to induce a broader and more efficient immune response against newly emerging strains has been investigated extensively. Studies in ferrets demonstrated that a recombinant H5N1 LAIV elicited a broad cross-protection against different H5N1 sublineages (Li et al., 1999, Suguitan et al., 2006). More recently, it was shown that immunization with LAIV H7N3 and H7N7 resulted in cross protection against the newly emerged H7N9 virus (Xu et al., 2013b).
The immunogenicity and extent of cross-protection have also been investigated for inactivated pandemic vaccines. In general, whole-virus inactivated vaccines induce robust responses after a single dose and may even protect against antigenetically mismatched viruses (Govorkova et al., 2006, Poland and Sambhara, 2008, Subbarao and Luke, 2007). In contrast, split-virus or subunit vaccines are less immunogenic, and more than one dose or the addition of an adjuvant are required to protect naïve individuals (Bresson et al., 2006, De Groot et al., 2013, Duan et al., 2014, Lin et al., 2006). The development of safe and efficacious adjuvants is of great relevance in the context of pandemic preparedness, since the use of adjuvants reduces the amount of antigen needed per dose (Clegg et al., 2014, Onishi et al., 2014). In addition, the development of adjuvanted seasonal vaccines is considered for seniors, who often respond poorly to the currently used non-adjuvanted inactivated vaccines (Ann et al., 2014, van den Brand et al., 2011).
A comprehensive overview of innovative vaccine approaches against influenza is beyond the scope of this review. Among the most promising candidates are virus-like particles (VLPs) composed of the M1 matrix protein together with the HA and NA proteins. Intranasal or intramuscular vaccination with such VLPs containing H5, H7 and H9 proteins, or the H1 protein alone, induced an immune response against all HAs present and protected ferrets from challenge with multiple avian influenza viruses (Mahmood et al., 2008, Pushko et al., 2011, Ross et al., 2009, Tretyakova et al., 2013). Different viral vector-based approaches have also been developed to the clinical trial stage. Among those, recombinant modified vaccinia virus Ankara (MVA) expressing different HA subtypes have demonstrated efficacy in ferrets and recent in first-in-man clinical trials have confirmed the immunogenicity in humans (Kreijtz et al., 2014, Kreijtz et al., 2010, Rimmelzwaan and Sutter, 2009). DNA- and more recently RNA-based vaccine candidates have great appeal in a pandemic context, since they can be produced rapidly and at large scale. DNA vaccines expressing single or multiple HA proteins alone or in combination with NA induce protective T and B cell responses in different animal models, especially in the context of prime-boost regimen with other vaccine candidates (Bragstad et al., 2011, Lin et al., 2012, Pillet et al., 2011, Rao et al., 2010, Suguitan et al., 2011). In a first proof-of-concept study, a PR8-HA-expressing RNA vaccine induced an immune response similar to approved influenza vaccines in ferrets and pigs (Petsch et al., 2012), indicating the feasibility of this approach. However, despite recent progress using in vivo electroporation or gene guns (Laddy et al., 2008, Yager et al., 2013), efficient DNA or RNA delivery remains a major obstacle for the implementation of this technology.
At this time, there are two classes of commercially available drugs against influenza, adamantane-derivatives, which inhibit the M2 ion channel, and neuraminidase inhibitors. In addition, the viral polymerase inhibitor favipiravir has received regulatory approval in Japan for use against influenza in 2014 (Furuta et al., 2013, Nagata et al., 2014). Early on, ferret studies demonstrated the rapid emergence of antiviral resistance against adamantanes and the transmission of drug-resistant strains without loss of virulence (Herlocher et al., 2003, Sweet et al., 1991), thereby predicting todays resistance of all circulating seasonal influenza strains (Bright et al., 2005, Bright et al., 2006). Nonetheless, adamantanes may still be useful in the context of a pandemic strain carrying a non-resistant M segment of animal origin (Nguyen et al., 2012). Ferret studies also demonstrated the efficacy of neuraminidase inhibitors against lethal infection with different H5N1 strains (Boltz et al., 2008a, Boltz et al., 2008b, Govorkova et al., 2007), which are now an integral part of pandemic preparedness strategies. After the emergence of oseltamivir-resistant strains, studies in ferrets have been used to characterize the impact of the mutations involved and to investigate the fitness cost associated with this resistance (Watanabe et al., 2013). Ferret studies thus remain an integral part in the development of new prophylactic and therapeutic strategies against influenza.
Paramyxoviruses
Henipaviruses
Hendra (HeV) and Nipah (NiV) viruses belong to the genus Henipavirus within the Paramyxoviridae family. Their natural hosts are fruit bats of the family Pteropodidae that can be found in South-East Asia, Australia and Africa (Hayman et al., 2008, Olson et al., 2002, Young et al., 1996). Infected bats shed the virus in urine, thereby infecting other species. Transmission to humans occurs through intermediate hosts like pigs (NiV) and horses (HeV) (Luby et al., 2009). Ephrin B2, which has been identified as the cellular receptor of henipaviruses, is highly conserved and present on endothelial cells, smooth muscle cells, and neurons (Gale et al., 2001), and thus thought to contribute to the broad species and tissue tropism (Bonaparte et al., 2005). In humans, the Henipavirus case fatality rate is approximately 70%. The disease starts with influenza-like symptoms such as fever, dry cough, sore throat, and lymphadenopathy, often leading to acute respiratory distress symptom (ARDS) (Hossain et al., 2008). There is a high incidence of neurologic symptoms and survivors often experience neurologic sequelae months and even years after the acute infection (O׳Sullivan et al., 1997, Playford et al., 2010, Tan et al., 2002, Wong et al., 2009).
Ferrets experimentally infected with NiV or HeV develop the full spectrum of disease seen in humans. First clinical signs occur 6–10 days after infection, starting with fever, coughing, nasal discharge, shortness of breath, and depression, sometimes followed by limb paralysis and meningitis (Bossart et al., 2009, Pallister et al., 2009, Pallister et al., 2011). Comparison of NiV strains isolated from patients in Bangladesh and Malaysia revealed differences in the amount of virus shed in oral secretions of infected ferrets, which may explain the increased human-to-human transmissions seen during outbreaks in Bangladesh (Clayton et al., 2012). However, more extensive pathogenesis and transmission studies are limited by the high containment requirements for the work with these viruses. A recently generated recombinant HeV expressing GFP or luciferase from an additional open reading frame retained the ability to cause fatal disease in ferrets, thus constituting a valuable tool to directly characterize dissemination and to visualize virus–host interactions (Marsh et al., 2013).
While there is currently no vaccine or antiviral treatment approved for humans, a HeV vaccine for horses based on soluble HeV glycoprotein has been available since 2012. Efficacy studies in ferrets were instrumental in the development of this vaccine (Bossart et al., 2005, Middleton et al., 2014, Pallister et al., 2011), and the analogous approach against NiV protected ferrets from NiV challenge for more than 12 months (Broder et al., 2013, Pallister et al., 2013). A vesicular stomatitis virus pseudotyped with the NiV glycoproteins was equally protective in ferrets, indicating that the development of an efficient and safe vaccine against these viruses is feasible (Mire et al., 2013). In addition, the potential of monoclonal antibody treatment has also been demonstrated in ferrets using a monoclonal antibody, which blocks the receptor-binding domain of the HeV and NiV G glycoproteins (Bossart et al., 2009), further validating ferrets as animal model for these viruses.
Pneumoviruses
Human respiratory syncytial virus (hRSV) and human metapneumovirus (hMPV) belong to the Pneumovirinae subfamily in the Paramyxoviridae family. They are one of the leading causes of respiratory disease in children worldwide, each year affecting more than 33 million children under the age of five as well as elderly and individuals with pre-existing respiratory conditions (Falsey et al., 2005, Nair et al., 2010, van den Hoogen et al., 2001). These viruses are transmitted via respiratory droplets or through direct contact with respiratory secretions (Hall et al., 1981). The infection is limited to respiratory epithelial cells (Lotz and Peebles, 2012), and the disease is mostly characterized by coughing, sneezing and fever. However, newborns, especially prematurely born infants, can progress to apnea, feeding difficulties, and periodic breathing (Collins and Graham, 2008, Dahlem et al., 2003, van den Hoogen et al., 2003). In elderly patients, the infection often leads to pneumonia and chronic pulmonary disease, asthma, and congestive heart failure (Falsey et al., 2003, Falsey et al., 2005). There is currently no vaccine against hRSV or hMPV, and the only approved antiviral drugs are ribavirin and a humanized monoclonal antibody directed against the RSV F glycoprotein (Johnson et al., 1997).
Ferrets are susceptible to hRSV and hMPV infection, and the viruses replicates to high titers in the nasal tissues (Coates and Chanock, 1962). However, hMPV-infected ferrets show no signs of disease (MacPhail et al., 2004). When animals were infected early in life, hRSV causes acute and chronic changes in the neural control of the airways that persist long after the virus was cleared (Colasurdo et al., 1998, Larsen and Colasurdo, 1999). Ferrets were also used to investigate the potential of recombinant adenoviruses expressing the RSV fusion and attachment glycoproteins individually or in combination. After intranasal administration, strong RSV-specific antibody responses were observed, and the animals were protected from subsequent RSV challenge (Hsu et al., 1994). Since replication in the lung only takes place in infant ferrets (Prince and Porter, 1976), their use as animal model for pneumovirus pathogenesis studies has been limited and little is known about the cellular receptors or mechanisms involved, even though ferrets reproduce the age-dependent differences in disease severity seen in humans.
Morbilliviruses
Ferrets are not susceptible to measles virus (MeV), the member of the Morbillivirus genus infecting humans and certain non-human primates. However, they are very sensitive to canine distemper virus (CDV), which infects a broad range of terrestrial and aquatic carnivores, and the disease reproduces all key elements seen in MeV-infected patients (Ludlow et al., 2014). Because of the high degree of genetic, structural, and functional conservation among morbilliviruses and the consistent course of disease caused by CDV in ferrets, this model is frequently used to characterize common morbillivirus pathogenesis mechanisms. Upon infection with a CDV wild type strain via the respiratory route, virus can first be isolated from peripheral blood mononuclear cells (PBMC) after 2–3 days, and most T and B lymphocytes in lymphatic organs are CDV-positive within one week (Pillet and von Messling, 2009). This coincides with a dramatic drop in white blood cell numbers and the loss of PBMC proliferation activity upon non-specific stimulation (von Messling et al., 2003). While the decrease in white blood cells is seen in all infections, the extent of inhibition of PBMC proliferation activity and of innate immune activation correlates with disease severity (Svitek and von Messling, 2007). The latter is controlled by the accessory V protein (Ramachandran et al., 2008, Rothlisberger et al., 2010), and a recent study in ferrets revealed that inhibition of STAT2 and mda5 signaling is essential for morbillivirus-mediated interference with the innate immune response (Svitek et al., 2014). After the signaling lymphocyte activation molecule (SLAM) was identified as morbillivirus immune cell receptor (Tatsuo et al., 2000, Tatsuo et al., 2001), pathogenesis studies with SLAM-blind viruses, first with CDV in ferrets and then with MeV in macaques, revealed that immune cell infection is essential for immunosuppression and clinical disease, thereby confirming the “immune cells first” hypothesis (Leonard et al., 2010, von Messling et al., 2005).
After amplification in lymphatic organs, the virus spreads to epithelial tissues throughout the body. If the immune response is not sufficient to control and subsequently clear the virus, the loss of epithelial integrity eventually leads to septicemia and death within three to five weeks after infection. The recently identified epithelial cell receptor nectin-4 (Muhlebach et al., 2011, Noyce et al., 2011) has enabled the generation of nectin-4-blind viruses, which subsequently not only illustrated the importance of epithelial cell infection for clinical disease but also demonstrated that acute immunosuppression requires immune but not epithelial cell infection (Frenzke et al., 2013, Sawatsky et al., 2012). Since the incidence of neuroinvasion in ferrets can reach 100% for some strains, they have also been used to investigate morbillivirus neuropathogenesis mechanisms. In addition to the previously known entry routes via the choroid plexus and small blood vessels, the anterograde infection along the olfactory nerves was identified as an additional entry pathway (Rudd et al., 2006). Comparative studies with different strains revealed a correlation with longer disease duration and less severe immunosuppression (Bonami et al., 2007).
The study of CDV in ferrets as a surrogate for MeV in primates has also been used to investigate the potential of novel vaccines and, more recently, an antiviral drug candidate (Krumm et al., 2014, Rouxel et al., 2009, Welter et al., 2000). The consistent pathogenesis together with the availability of robust reverse genetics systems for several vaccine and wild type strains thus make the CDV-ferret model an attractive system to characterize virus-host interactions in the context of lethal disease outcome or survival, and to investigate the impact of interventions at different stages of disease.
Coronaviruses
In addition to viruses causing a mild upper respiratory tract infection, the Coronavirinae subfamily also includes members that lead to severe acute respiratory disease with mortality rates of around 10% for severe acute respiratory syndrome (SARS)-CoV and even higher for Middle East respiratory syndrome (MERS)-CoV (Butler, 2012, Hui et al., 2014). Because of the respiratory tropism, the susceptibility of ferrets was evaluated. In the case of SARS-CoV, the virus was found to replicated efficiently in the upper and lower respiratory tract, and the animals developed clinical disease characterized by nasal discharge, sneezing, fever, and virus shedding including contact transmission to naïve cage mates after intratracheal infection (Chu et al., 2008, Martina et al., 2003). However, no clinical signs were seen after intranasal infection in a different study (Weingartl et al., 2004). This variability may be due to the route of infection, and the age, gender, and genetics of the animals are likely additional contributing factors.
In humans, the SARS-CoV tropism reflects the distribution of the cellular receptor angiotensin-converting enzyme (ACE) 2 (Drosten et al., 2003, Li et al., 2003) in lung alveolar epithelial cells, enterocytes of the small intestine and tubular cells of the kidney (Ding et al., 2004, Gu et al., 2005, Hamming et al., 2004). The disease can range from mild respiratory signs to severe respiratory failure. After incubation period of 2–14 days patients initially present with influenza-like symptoms, fever, and cough that can progress to atypic pneumonia and respiratory failure (Peiris et al., 2003). The virus can also spread to multiple organs including the gastrointestinal and urinary tract causing a systemic disease. Even though it has been shown that ferret ACE2 interacts efficiently the SARS-CoV receptor-binding spike (S) protein (Zamoto et al., 2006), the tropism of SARS-CoV in ferrets does not match the receptor distribution (Gramberg et al., 2005), which may explain the differences in clinical manifestation observed.
Nevertheless, the ferret represents a useful animal model for the safety and efficacy assessment of SARS-CoV vaccine candidates and therapeutic approaches. While a MVA-based vaccine expressing the SARS-CoV S protein elicited a strong neutralizing immune response and protected the animals from disease, inflammatory lesions were found in the liver of the vaccinated animals (Weingartl et al., 2004). Formalin-inactivated whole-virus vaccines as well as an S-expressing adenovirus-based vaccine also induced neutralizing antibodies and no liver pathology was noted, but the extent of protection varied, indicating that that a combination of vaccine strategies may be required for effective protection (Darnell et al., 2007, See et al., 2008). Prophylactic treatment with a monoclonal antibody reduced the replication of SARS-CoV in the lungs of infected ferrets up to one thousand-fold and may thus be useful in protecting contacts thereby limiting the spread of the disease (ter Meulen et al., 2004).
In the case of MERS-CoV, which also causes a severe pulmonary disease in humans, similar to SARS-CoV (Zaki et al., 2012), ferrets were not found to be susceptible. Even though MERS-CoV and SARS-CoV are both members of the genus Betacoronavirus, they differ in their receptor usage. MERS-CoV uses dipeptidyl peptidase (DPP) 4 for cell entry (Raj et al., 2013), which can also be found on ferret cells, but the sequence differences between the human and ferret DPP4 protein prevent binding of MERS-CoV (van Doremalen et al., 2014) explaining the lack of infection in this species.
Others
Even though rabies pathogenesis is mostly studied in dogs, raccoons, or foxes, the protective efficacy of a commercial, inactivated rabies vaccine has also been demonstrated in ferrets (Rupprecht et al., 1990). In the context of a pathogenesis study, experimentally infected ferrets were assessed for susceptibility, incubation period, morbidity, clinical signs, seroconversion, and virus shedding. Interestingly, 17 out of 55 ferrets survived the infection, and rabies virus was isolated only from one of these animals (Niezgoda et al., 1997). Survival of ferrets experimentally infected with rabies virus was also reported in a different study (Hamir et al., 2011), indicating that they might be more resistant than other carnivores. Ferrets were also used to characterize newly discovered lyssaviruses with zoonotic potential and to evaluate the efficacy and cross-protection of available rabies vaccines (Hanlon et al., 2005, Vos et al., 2004).
The potential of ferrets as an animal model has been evaluated for other viral diseases, including mumps virus, simian virus 5, canine or human parainfluenza viruses, and rubella virus. Two recent studies demonstrated that mumps virus-infected ferrets mounted robust humoral and cellular immune response but developed no clinical signs and no virus was shed, indicating that the use of the model is limited (Parker et al., 2013, Xu et al., 2013a). Ferrets infected with simian virus 5 or canine and human parainfluenza viruses, which generally cause a mild and transient disease in their natural hosts, develop antibody responses and histopathological changes indicative of an infection in the upper respiratory tract similar to those seen in other species, and may thus be an attractive model for the development of vaccines or antivirals (Capraro et al., 2008, Durchfeld et al., 1991, Mascoli et al., 1976, Mascoli et al., 1975). Finally, during the late sixties, the use of ferrets as an animal model for neonatal rubella was explored but this was ultimately unsuccessful (Elizan et al., 1969, Fabiyi et al., 1967, Rorke et al., 1968).
Conclusion
Ferrets have been used as animal models in viral research since the beginning of the 20th century, starting with influenza and, as their susceptibility for human respiratory viruses became increasingly apparent, followed by an ever increasing number of additional pathogens ( Table 1). Even though their housing requirements are more elaborate than those for rodents, ferrets can still be accommodated in most animal facilities, and animals can be purchased from several commercial sources. The greatest disadvantage of the ferret as an animal model remains the relative lack of species-specific reagents. Although international efforts have been made to improve this situation by generating ferret-specific tools and by identifying cross-reactive reagents (Fang et al., 2010, Rutigliano et al., 2008), ferret immune responses are still poorly characterized. In addition, the potential of the model to characterize the contribution of opportunistic infections and different routes of inoculation, the dynamics of aerosol transmission, and infections in the context of immunosuppression or co-morbidities is just beginning to be explored (Belser et al., 2014, Gustin et al., 2013, Huber and McCullers, 2006, Koster et al., 2012, McCullers et al., 2010, Peltola et al., 2006). Because of its great public health relevance, most of the studies to date have focused on influenza viruses, but an extension to other pathogens is likely to follow in the near future.
Table 1.
Research topics studied in ferrets.
| Viruses | Transmission | Pathogenesis | Neurovirulence | Immune response | Vaccination strategies | Antivirals and treatment |
|---|---|---|---|---|---|---|
| Orthomyxoviruses | ||||||
| Influenza A viruses | + | + | + | + | + | + |
| Paramyxoviruses | ||||||
| Morbilliviruses | ||||||
| Canine distemper virus (CDV) | + | + | + | + | + | + |
| Measles virus (MeV) | Ø | Ø | Ø | Ø | Ø | Ø |
| Henipaviruses | ||||||
| Nipah virus (NiV) | − | + | + | − | + | + |
| Hendra virus (HeV) | − | + | − | − | + | + |
| Rubulaviruses | ||||||
| Mumps virus (MuV) | − | + | − | + | − | − |
| Human parainfluenza virus (hPIV) 2 | − | + | − | + | − | − |
| Simian virus (SV) 5 | − | − | − | + | − | − |
| Respiroviruses | ||||||
| Human parainfluenza virus (hPIV) 1, 3 | − | + | − | + | − | − |
| Pneumoviruses | ||||||
| Human metapneumovirus (hMPV) | − | + | − | − | − | − |
| Human respiratory syncytial virus (hRSV) | − | + | + | − | + | − |
| Rhabdoviruses | ||||||
| Lyssaviruses | ||||||
| Rabies virus | − | + | + | − | + | − |
| Togaviruses | ||||||
| Rubiviruses | ||||||
| Rubella virus | + (vertical) | − | − | − | − | − |
| Coronaviridae | ||||||
| Betacoronaviruses | ||||||
| SARS-Coronavirus (SARS-CoV) | + | + | − | + | + | − |
| MERS-Coronavirus (MERS-CoV) | Ø | Ø | Ø | Ø | Ø | Ø |
+: published.
−: not published.
Ø: no infection.
The completion of the ferret genome and the publication of the ferret transcriptome (Bruder et al., 2010, Peng et al., 2014) also open new avenues of experimentation. Not only is it possible to identify transcribed mRNA or activated genes at a given time point of any viral infection, but the ferret genome information also facilitates the development of species-specific genomic and proteomic tools and the mapping of signaling pathways. After succeeding with somatic cell nuclear transfer (Li et al., 2006), the CFTR-knockout ferret as a cystic fibrosis model (Sun et al., 2010) provided proof-of-concept for the generation of transgenic animals with specific phenotypes, and technical advances in the transgenics field such as the use of sleeping beauty transposons (Ivics et al., 2014) and the CRISPR-Cas system (Hai et al., 2014, Tang et al., 2015), will put transgenic ferrets within the reach of the infectious disease research community. As new viruses continue to emerge and additional immunologic, genomic, and proteomic tools for ferrets become available, the importance of this animal model for infectious disease research will likely continue to increase.
References
- Ann J., Samant M., Rheaume C., Dumas C., Beaulieu E., Morasse A., Mallett C., Hamelin M.E., Papadopoulou B., Boivin G. Adjuvanted inactivated influenza A(H3N2) vaccines induce stronger immunogenicity in mice and confer higher protection in ferrets than unadjuvanted inactivated vaccines. Vaccine. 2014;32(43):5730–5739. doi: 10.1016/j.vaccine.2014.08.029. [DOI] [PubMed] [Google Scholar]
- Ball R.S. Husbandry and management of the domestic ferret. Lab Anim (NY) 2002;31(5):37–42. doi: 10.1038/5000157. [DOI] [PubMed] [Google Scholar]
- Belser J.A., Gustin K.M., Katz J.M., Maines T.R., Tumpey T.M. Influenza virus infectivity and virulence following ocular-only aerosol inoculation of ferrets. J. Virol. 2014;88(17):9647–9654. doi: 10.1128/JVI.01067-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belser J.A., Gustin K.M., Maines T.R., Blau D.M., Zaki S.R., Katz J.M., Tumpey T.M. Pathogenesis and transmission of triple-reassortant swine H1N1 influenza viruses isolated before the 2009 H1N1 pandemic. J. Virol. 2011;85(4):1563–1572. doi: 10.1128/JVI.02231-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belser J.A., Gustin K.M., Maines T.R., Pantin-Jackwood M.J., Katz J.M., Tumpey T.M. Influenza virus respiratory infection and transmission following ocular inoculation in ferrets. PLoS Pathog. 2012;8(3):e1002569. doi: 10.1371/journal.ppat.1002569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belser J.A., Gustin K.M., Pearce M.B., Maines T.R., Zeng H., Pappas C., Sun X., Carney P.J., Villanueva J.M., Stevens J., Katz J.M., Tumpey T.M. Pathogenesis and transmission of avian influenza A (H7N9) virus in ferrets and mice. Nature. 2013;501(7468):556–559. doi: 10.1038/nature12391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belser J.A., Szretter K.J., Katz J.M., Tumpey T.M. Use of animal models to understand the pandemic potential of highly pathogenic avian influenza viruses. Adv. Virus Res. 2009;73:55–97. doi: 10.1016/S0065-3527(09)73002-7. [DOI] [PubMed] [Google Scholar]
- Bertram S., Glowacka I., Steffen I., Kuhl A., Pohlmann S. Novel insights into proteolytic cleavage of influenza virus hemagglutinin. Rev. Med. Virol. 2010;20(5):298–310. doi: 10.1002/rmv.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bixler H., Ellis C. Ferret care and husbandry. Veterinary clinics of North America. Exotic Anim. Pract. 2004;7:227–255. doi: 10.1016/j.cvex.2004.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodewes R., de Mutsert G., van der Klis F.R., Ventresca M., Wilks S., Smith D.J., Koopmans M., Fouchier R.A., Osterhaus A.D., Rimmelzwaan G.F. Prevalence of antibodies against seasonal influenza A and B viruses in children in Netherlands. Clin. Vaccine Immunol. 2011;18(3):469–476. doi: 10.1128/CVI.00396-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodewes R., Kreijtz J.H., van Amerongen G., Hillaire M.L., Vogelzang-van Trierum S.E., Nieuwkoop N.J., van Run P., Kuiken T., Fouchier R.A., Osterhaus A.D., Rimmelzwaan G.F. Infection of the upper respiratory tract with seasonal influenza A(H3N2) virus induces protective immunity in ferrets against infection with A(H1N1)pdm09 virus after intranasal, but not intratracheal, inoculation. J. Virol. 2013;87(8):4293–4301. doi: 10.1128/JVI.02536-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boltz D.A., Ilyushina N.A., Arnold C.S., Babu Y.S., Webster R.G., Govorkova E.A. Intramuscularly administered neuraminidase inhibitor peramivir is effective against lethal H5N1 influenza virus in mice. Antiviral Res. 2008;80(2):150–157. doi: 10.1016/j.antiviral.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boltz D.A., Rehg J.E., McClaren J., Webster R.G., Govorkova E.A. Oseltamivir prophylactic regimens prevent H5N1 influenza morbidity and mortality in a ferret model. J. Infect. Dis. 2008;197(9):1315–1323. doi: 10.1086/586711. [DOI] [PubMed] [Google Scholar]
- Bonami F., Rudd P.A., von Messling V. Disease duration determines canine distemper virus neurovirulence. J. Virol. 2007;81(21):12066–12070. doi: 10.1128/JVI.00818-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaparte M.I., Dimitrov A.S., Bossart K.N., Crameri G., Mungall B.A., Bishop K.A., Choudhry V., Dimitrov D.S., Wang L.F., Eaton B.T., Broder C.C. Ephrin-B2 ligand is a functional receptor for Hendra virus and Nipah virus. Proc. Natl. Acad. Sci. USA. 2005;102(30):10652–10657. doi: 10.1073/pnas.0504887102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossart K.N., Crameri G., Dimitrov A.S., Mungall B.A., Feng Y.R., Patch J.R., Choudhary A., Wang L.F., Eaton B.T., Broder C.C. Receptor binding, fusion inhibition, and induction of cross-reactive neutralizing antibodies by a soluble G glycoprotein of Hendra virus. J. Virol. 2005;79(11):6690–6702. doi: 10.1128/JVI.79.11.6690-6702.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossart K.N., Zhu Z., Middleton D., Klippel J., Crameri G., Bingham J., McEachern J.A., Green D., Hancock T.J., Chan Y.P., Hickey A.C., Dimitrov D.S., Wang L.F., Broder C.C. A neutralizing human monoclonal antibody protects against lethal disease in a new ferret model of acute nipah virus infection. PLoS Pathog. 2009;5(10):e1000642. doi: 10.1371/journal.ppat.1000642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvier N.M., Lowen A.C. Animal models for influenza virus pathogenesis and transmission. Viruses. 2010;2(8):1530–1563. doi: 10.3390/v20801530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragstad K., Martel C.J., Thomsen J.S., Jensen K.L., Nielsen L.P., Aasted B., Fomsgaard A. Pandemic influenza 1918 H1N1 and 1968 H3N2 DNA vaccines induce cross-reactive immunity in ferrets against infection with viruses drifted for decades. Influenza Other Respir. Viruses. 2011;5(1):13–23. doi: 10.1111/j.1750-2659.2010.00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brankston G., Gitterman L., Hirji Z., Lemieux C., Gardam M. Transmission of influenza A in human beings. Lancet Infect. Dis. 2007;7(4):257–265. doi: 10.1016/S1473-3099(07)70029-4. [DOI] [PubMed] [Google Scholar]
- Bresson J.L., Perronne C., Launay O., Gerdil C., Saville M., Wood J., Hoschler K., Zambon M.C. Safety and immunogenicity of an inactivated split-virion influenza A/Vietnam/1194/2004 (H5N1) vaccine: phase I randomised trial. Lancet. 2006;367(9523):1657–1664. doi: 10.1016/S0140-6736(06)68656-X. [DOI] [PubMed] [Google Scholar]
- Bright R.A., Medina M.J., Xu X., Perez-Oronoz G., Wallis T.R., Davis X.M., Povinelli L., Cox N.J., Klimov A.I. Incidence of adamantane resistance among influenza A (H3N2) viruses isolated worldwide from 1994 to 2005: a cause for concern. Lancet. 2005;366(9492):1175–1181. doi: 10.1016/S0140-6736(05)67338-2. [DOI] [PubMed] [Google Scholar]
- Bright R.A., Shay D.K., Shu B., Cox N.J., Klimov A.I. Adamantane resistance among influenza A viruses isolated early during the 2005–2006 influenza season in the United States. JAMA. 2006;295(8):891–894. doi: 10.1001/jama.295.8.joc60020. [DOI] [PubMed] [Google Scholar]
- Broder C.C., Xu K., Nikolov D.B., Zhu Z., Dimitrov D.S., Middleton D., Pallister J., Geisbert T.W., Bossart K.N., Wang L.F. A treatment for and vaccine against the deadly Hendra and Nipah viruses. Antiviral Res. 2013;100(1):8–13. doi: 10.1016/j.antiviral.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruder C.E., Yao S., Larson F., Camp J.V., Tapp R., McBrayer A., Powers N., Granda W.V., Jonsson C.B. Transcriptome sequencing and development of an expression microarray platform for the domestic ferret. BMC Genomics. 2010;11:251. doi: 10.1186/1471-2164-11-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler D. Clusters of coronavirus cases put scientists on alert. Nature. 2012;492(7428):166–167. doi: 10.1038/492166a. [DOI] [PubMed] [Google Scholar]
- Cameron C.M., Cameron M.J., Bermejo-Martin J.F., Ran L., Xu L., Turner P.V., Ran R., Danesh A., Fang Y., Chan P.K., Mytle N., Sullivan T.J., Collins T.L., Johnson M.G., Medina J.C., Rowe T., Kelvin D.J. Gene expression analysis of host innate immune responses during Lethal H5N1 infection in ferrets. J. Virol. 2008;82(22):11308–11317. doi: 10.1128/JVI.00691-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capraro G.A., Johnson J.B., Kock N.D., Parks G.D. Virus growth and antibody responses following respiratory tract infection of ferrets and mice with WT and P/V mutants of the paramyxovirus Simian Virus 5. Virology. 2008;376(2):416–428. doi: 10.1016/j.virol.2008.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Bright R.A., Subbarao K., Smith C., Cox N.J., Katz J.M., Matsuoka Y. Polygenic virulence factors involved in pathogenesis of 1997 Hong Kong H5N1 influenza viruses in mice. Virus Res. 2007;128(1–2):159–163. doi: 10.1016/j.virusres.2007.04.017. [DOI] [PubMed] [Google Scholar]
- Chu Y.K., Ali G.D., Jia F., Li Q., Kelvin D., Couch R.C., Harrod K.S., Hutt J.A., Cameron C., Weiss S.R., Jonsson C.B. The SARS-CoV ferret model in an infection-challenge study. Virology. 2008;374(1):151–163. doi: 10.1016/j.virol.2007.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton B.A., Middleton D., Bergfeld J., Haining J., Arkinstall R., Wang L., Marsh G.A. Transmission routes for nipah virus from Malaysia and Bangladesh. Emerg. Infect. Dis. 2012;18(12):1983–1993. doi: 10.3201/eid1812.120875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg C.H., Roque R., Perrone L.A., Rininger J.A., Bowen R., Reed S.G. GLA-AF, an emulsion-free vaccine adjuvant for pandemic influenza. PLoS One. 2014;9(2):e88979. doi: 10.1371/journal.pone.0088979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clingerman K.J., Fox J.G., Walke M., National Agricultural Library (U.S.), and Massachusetts Institute of Technology . Bibliographies and Literature of Agriculture U.S. Department of Agriculture Massachusetts Institute of Technology; Beltsville, MD, Cambridge, MA: 1991. Ferrets as Laboratory Animals: a Bibliography. [Google Scholar]
- Coates H.V., Chanock R.M. Experimental infection with respiratory syncytial virus in several species of animals. Amin. J. Hyg. 1962;76:302–312. doi: 10.1093/oxfordjournals.aje.a120285. [DOI] [PubMed] [Google Scholar]
- Colasurdo G.N., Hemming V.G., Prince G.A., Gelfand A.S., Loader J.E., Larsen G.L. Human respiratory syncytial virus produces prolonged alterations of neural control in airways of developing ferrets. Am. J. Respir. Crit. Care Med. 1998;157(5, Part 1):1506–1511. doi: 10.1164/ajrccm.157.5.9705026. [DOI] [PubMed] [Google Scholar]
- Collins P.L., Graham B.S. Viral and host factors in human respiratory syncytial virus pathogenesis. J. Virol. 2008;82(5):2040–2055. doi: 10.1128/JVI.01625-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlem P., van Aalderen W.M., Hamaker M.E., Dijkgraaf M.G., Bos A.P. Incidence and short-term outcome of acute lung injury in mechanically ventilated children. Eur. Respir. J. 2003;22(6):980–985. doi: 10.1183/09031936.03.00003303. [DOI] [PubMed] [Google Scholar]
- Darnell M.E., Plant E.P., Watanabe H., Byrum R., St. Claire M., Ward J.M., Taylor D.R. Severe acute respiratory syndrome coronavirus infection in vaccinated ferrets. J. Infect. Dis. 2007;196(9):1329–1338. doi: 10.1086/522431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groot A.S., Ardito M., Terry F., Levitz L., Ross T., Moise L., Martin W. Low immunogenicity predicted for emerging avian-origin H7N9: implication for influenza vaccine design. Hum. Vaccin. Immunother. 2013;9(5):950–956. doi: 10.4161/hv.24939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong M.D., Simmons C.P., Thanh T.T., Hien V.M., Smith G.J., Chau T.N., Hoang D.M., Chau N.V., Khanh T.H., Dong V.C., Qui P.T., Cam B.V., Ha do Q., Guan Y., Peiris J.S., Chinh N.T., Hien T.T., Farrar J. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat. Med. 2006;12(10):1203–1207. doi: 10.1038/nm1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y., He L., Zhang Q., Huang Z., Che X., Hou J., Wang H., Shen H., Qiu L., Li Z., Geng J., Cai J., Han H., Li X., Kang W., Weng D., Liang P., Jiang S. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J. Pathol. 2004;203(2):622–630. doi: 10.1002/path.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drosten C., Gunther S., Preiser W., van der Werf S., Brodt H.R., Becker S., Rabenau H., Panning M., Kolesnikova L., Fouchier R.A., Berger A., Burguiere A.M., Cinatl J., Eickmann M., Escriou N., Grywna K., Kramme S., Manuguerra J.C., Muller S., Rickerts V., Sturmer M., Vieth S., Klenk H.D., Osterhaus A.D., Schmitz H., Doerr H.W. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348(20):1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- Duan Y., Gu H., Chen R., Zhao Z., Zhang L., Xing L., Lai C., Zhang P., Li Z., Zhang K., Wang Z., Zhang S., Wang X., Yang P. Response of mice and ferrets to a monovalent influenza A (H7N9) split vaccine. PLoS One. 2014;9(6):e99322. doi: 10.1371/journal.pone.0099322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durchfeld B., Baumgartner W., Krakowka S. Intranasal infection of ferrets (Mustela putorius furo) with canine parainfluenza virus. Zentralbl. Veterinarmed. B. 1991;38(7):505–512. doi: 10.1111/j.1439-0450.1991.tb00904.x. [DOI] [PubMed] [Google Scholar]
- Elizan T.S., Fabiyi A., Sever J.L. Experimental teratogenesis in ferrets using Rubella virus. J. Mt. Sinai Hosp. N. Y. 1969;36(2):103–107. [PubMed] [Google Scholar]
- Fabiyi A., Gitnick G.L., Sever J.L. Chronic rubella virus infection in the ferret (Mustela putorius fero) puppy. Proc. Soc. Exp. Biol. Med. 1967;125(3):766–771. doi: 10.3181/00379727-125-32200. [DOI] [PubMed] [Google Scholar]
- Falsey A.R., Erdman D., Anderson L.J., Walsh E.E. Human metapneumovirus infections in young and elderly adults. J. Infect. Dis. 2003;187(5):785–790. doi: 10.1086/367901. [DOI] [PubMed] [Google Scholar]
- Falsey A.R., Hennessey P.A., Formica M.A., Cox C., Walsh E.E. Respiratory syncytial virus infection in elderly and high-risk adults. N. Engl. J. Med. 2005;352(17):1749–1759. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- Fan S., Zhou L., Wu D., Gao X., Pei E., Wang T., Gao Y., Xia X. A novel highly pathogenic H5N8 avian influenza virus isolated from a wild duck in China. Influenza Other Respir. Viruses. 2014;8(6):646–653. doi: 10.1111/irv.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y., Rowe T., Leon A.J., Banner D., Danesh A., Xu L., Ran L., Bosinger S.E., Guan Y., Chen H., Cameron C.C., Cameron M.J., Kelvin D.J. Molecular characterization of in vivo adjuvant activity in ferrets vaccinated against influenza virus. J. Virol. 2010;84(17):8369–8388. doi: 10.1128/JVI.02305-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonville J.M., Wilks S.H., James S.L., Fox A., Ventresca M., Aban M., Xue L., Jones T.C., Le N.M., Pham Q.T., Tran N.D., Wong Y., Mosterin A., Katzelnick L.C., Labonte D., Le T.T., van der Net G., Skepner E., Russell C.A., Kaplan T.D., Rimmelzwaan G.F., Masurel N., de Jong J.C., Palache A., Beyer W.E., Le Q.M., Nguyen T.H., Wertheim H.F., Hurt A.C., Osterhaus A.D., Barr I.G., Fouchier R.A., Horby P.W., Smith D.J. Antibody landscapes after influenza virus infection or vaccination. Science. 2014;346(6212):996–1000. doi: 10.1126/science.1256427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J.G. 2nd ed. Williams & Wilkins; Baltimore: 1998. Biology and Diseases of the Ferret. [Google Scholar]
- Frenzke M., Sawatsky B., Wong X.X., Delpeut S., Mateo M., Cattaneo R., von Messling V. Nectin-4-dependent measles virus spread to the cynomolgus monkey tracheal epithelium: role of infected immune cells infiltrating the lamina propria. J. Virol. 2013;87(5):2526–2534. doi: 10.1128/JVI.03037-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y., Gowen B.B., Takahashi K., Shiraki K., Smee D.F., Barnard D.L. Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antiviral Res. 2013;100(2):446–454. doi: 10.1016/j.antiviral.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale N.W., Baluk P., Pan L., Kwan M., Holash J., DeChiara T.M., McDonald D.M., Yancopoulos G.D. Ephrin-B2 selectively marks arterial vessels and neovascularization sites in the adult, with expression in both endothelial and smooth-muscle cells. Dev. Biol. 2001;230(2):151–160. doi: 10.1006/dbio.2000.0112. [DOI] [PubMed] [Google Scholar]
- Gambotto A., Barratt-Boyes S.M., de Jong M.D., Neumann G., Kawaoka Y. Human infection with highly pathogenic H5N1 influenza virus. Lancet. 2008;371(9622):1464–1475. doi: 10.1016/S0140-6736(08)60627-3. [DOI] [PubMed] [Google Scholar]
- Gerhard W. The role of the antibody response in influenza virus infection. Curr. Top. Microbiol. Immunol. 2001;260:171–190. doi: 10.1007/978-3-662-05783-4_9. [DOI] [PubMed] [Google Scholar]
- Govorkova E.A., Ilyushina N.A., Boltz D.A., Douglas A., Yilmaz N., Webster R.G. Efficacy of oseltamivir therapy in ferrets inoculated with different clades of H5N1 influenza virus. Antimicrob. Agents Chemother. 2007;51(4):1414–1424. doi: 10.1128/AAC.01312-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govorkova E.A., Webby R.J., Humberd J., Seiler J.P., Webster R.G. Immunization with reverse-genetics-produced H5N1 influenza vaccine protects ferrets against homologous and heterologous challenge. J. Infect. Dis. 2006;194(2):159–167. doi: 10.1086/505225. [DOI] [PubMed] [Google Scholar]
- Gramberg T., Hofmann H., Moller P., Lalor P.F., Marzi A., Geier M., Krumbiegel M., Winkler T., Kirchhoff F., Adams D.H., Becker S., Munch J., Pohlmann S. LSECtin interacts with filovirus glycoproteins and the spike protein of SARS coronavirus. Virology. 2005;340(2):224–236. doi: 10.1016/j.virol.2005.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J., Gong E., Zhang B., Zheng J., Gao Z., Zhong Y., Zou W., Zhan J., Wang S., Xie Z., Zhuang H., Wu B., Zhong H., Shao H., Fang W., Gao D., Pei F., Li X., He Z., Xu D., Shi X., Anderson V.M., Leong A.S. Multiple organ infection and the pathogenesis of SARS. J. Exp. Med. 2005;202(3):415–424. doi: 10.1084/jem.20050828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustin K.M., Katz J.M., Tumpey T.M., Maines T.R. Comparison of the levels of infectious virus in respirable aerosols exhaled by ferrets infected with influenza viruses exhibiting diverse transmissibility phenotypes. J. Virol. 2013;87(14):7864–7873. doi: 10.1128/JVI.00719-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hai T., Teng F., Guo R., Li W., Zhou Q. One-step generation of knockout pigs by zygote injection of CRISPR/Cas system. Cell Res. 2014;24(3):372–375. doi: 10.1038/cr.2014.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall C.B., Douglas R.G., Jr., Schnabel K.C., Geiman J.M. Infectivity of respiratory syncytial virus by various routes of inoculation. Infect. Immun. 1981;33(3):779–783. doi: 10.1128/iai.33.3.779-783.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamir A.N., Niezgoda M., Rupprecht C.E. Recovery from and clearance of rabies virus in a domestic ferret. J. Am. Assoc. Lab. Anim. Sci. 2011;50(2):248–251. [PMC free article] [PubMed] [Google Scholar]
- Hamming I., Timens W., Bulthuis M.L., Lely A.T., Navis G., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon C.A., Kuzmin I.V., Blanton J.D., Weldon W.C., Manangan J.S., Rupprecht C.E. Efficacy of rabies biologics against new lyssaviruses from Eurasia. Virus Res. 2005;111(1):44–54. doi: 10.1016/j.virusres.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Hatta M., Gao P., Halfmann P., Kawaoka Y. Molecular basis for high virulence of Hong Kong H5N1 influenza A viruses. Science. 2001;293(5536):1840–1842. doi: 10.1126/science.1062882. [DOI] [PubMed] [Google Scholar]
- Hayman D.T., Suu-Ire R., Breed A.C., McEachern J.A., Wang L., Wood J.L., Cunningham A.A. Evidence of henipavirus infection in West African fruit bats. PLoS One. 2008;3(7):e2739. doi: 10.1371/journal.pone.0002739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herfst S., Schrauwen E.J., Linster M., Chutinimitkul S., de Wit E., Munster V.J., Sorrell E.M., Bestebroer T.M., Burke D.F., Smith D.J., Rimmelzwaan G.F., Osterhaus A.D., Fouchier R.A. Airborne transmission of influenza A/H5N1 virus between ferrets. Science. 2012;336(6088):1534–1541. doi: 10.1126/science.1213362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlocher M.L., Truscon R., Fenton R., Klimov A., Elias S., Ohmit S.E., Monto A.S. Assessment of development of resistance to antivirals in the ferret model of influenza virus infection. J. Infect. Dis. 2003;188(9):1355–1361. doi: 10.1086/379049. [DOI] [PubMed] [Google Scholar]
- Hoschler K., Thompson C., Casas I., Ellis J., Galiano M., Andrews N., Zambon M. Population susceptibility to North American and Eurasian swine influenza viruses in England, at three time points between 2004 and 2011. Euro Surveill. 2013;18(36):pii=20578. doi: 10.2807/1560-7917.es2013.18.36.20578. [DOI] [PubMed] [Google Scholar]
- Hossain M.J., Gurley E.S., Montgomery J.M., Bell M., Carroll D.S., Hsu V.P., Formenty P., Croisier A., Bertherat E., Faiz M.A., Azad A.K., Islam R., Molla M.A., Ksiazek T.G., Rota P.A., Comer J.A., Rollin P.E., Luby S.P., Breiman R.F. Clinical presentation of nipah virus infection in Bangladesh. Clin. Infect. Dis. 2008;46(7):977–984. doi: 10.1086/529147. [DOI] [PubMed] [Google Scholar]
- Hsu K.H., Lubeck M.D., Bhat B.M., Bhat R.A., Kostek B., Selling B.H., Mizutani S., Davis A.R., Hung P.P. Efficacy of adenovirus-vectored respiratory syncytial virus vaccines in a new ferret model. Vaccine. 1994;12(7):607–612. doi: 10.1016/0264-410x(94)90264-x. [DOI] [PubMed] [Google Scholar]
- Huang S.S., Banner D., Fang Y., Ng D.C., Kanagasabai T., Kelvin D.J., Kelvin A.A. Comparative analyses of pandemic H1N1 and seasonal H1N1, H3N2, and influenza B infections depict distinct clinical pictures in ferrets. PLoS One. 2011;6(11):e27512. doi: 10.1371/journal.pone.0027512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber V.C., McCullers J.A. Live attenuated influenza vaccine is safe and immunogenic in immunocompromised ferrets. J. Infect. Dis. 2006;193(5):677–684. doi: 10.1086/500247. [DOI] [PubMed] [Google Scholar]
- Hui D.S., Memish Z.A., Zumla A. Severe acute respiratory syndrome vs. the Middle East respiratory syndrome. Curr. Opin. Pulm. Med. 2014;20(3):233–241. doi: 10.1097/MCP.0000000000000046. [DOI] [PubMed] [Google Scholar]
- Imai M., Watanabe T., Hatta M., Das S.C., Ozawa M., Shinya K., Zhong G., Hanson A., Katsura H., Watanabe S., Li C., Kawakami E., Yamada S., Kiso M., Suzuki Y., Maher E.A., Neumann G., Kawaoka Y. Experimental adaptation of an influenza H5 HA confers respiratory droplet transmission to a reassortant H5 HA/H1N1 virus in ferrets. Nature. 2012;486(7403):420–428. doi: 10.1038/nature10831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivics Z., Hiripi L., Hoffmann O.I., Mates L., Yau T.Y., Bashir S., Zidek V., Landa V., Geurts A., Pravenec M., Rulicke T., Bosze Z., Izsvak Z. Germline transgenesis in rabbits by pronuclear microinjection of Sleeping Beauty transposons. Nat. Protoc. 2014;9(4):794–809. doi: 10.1038/nprot.2014.009. [DOI] [PubMed] [Google Scholar]
- Johnson-Delaney C.A., Orosz S.E. Ferret respiratory system: clinical anatomy, physiology, and disease. Vet. Clin. N. Am. Exot. Anim. Pract. 2011;14(2):357–367. doi: 10.1016/j.cvex.2011.03.001. vii. [DOI] [PubMed] [Google Scholar]
- Johnson N.P., Mueller J. Updating the accounts: global mortality of the 1918–1920 “Spanish” influenza pandemic. Bull. Hist. Med. 2002;76(1):105–115. doi: 10.1353/bhm.2002.0022. [DOI] [PubMed] [Google Scholar]
- Johnson S., Oliver C., Prince G.A., Hemming V.G., Pfarr D.S., Wang S.C., Dormitzer M., O׳Grady J., Koenig S., Tamura J.K., Woods R., Bansal G., Couchenour D., Tsao E., Hall W.C., Young J.F. Development of a humanized monoclonal antibody (MEDI-493) with potent in vitro and in vivo activity against respiratory syncytial virus. J. Infect. Dis. 1997;176(5):1215–1224. doi: 10.1086/514115. [DOI] [PubMed] [Google Scholar]
- Kang Y.M., Song B.M., Lee J.S., Kim H.S., Seo S.H. Pandemic H1N1 influenza virus causes a stronger inflammatory response than seasonal H1N1 influenza virus in ferrets. Arch. Virol. 2011;156(5):759–767. doi: 10.1007/s00705-010-0914-7. [DOI] [PubMed] [Google Scholar]
- Karlsson E.A., Ip H.S., Hall J.S., Yoon S.W., Johnson J., Beck M.A., Webby R.J., Schultz-Cherry S. Respiratory transmission of an avian H3N8 influenza virus isolated from a harbour seal. Nat. Commun. 2014;5:4791. doi: 10.1038/ncomms5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.-I., Pascua P.N.Q., Kwon H.-I., Lim G.-J., Kim E.-H., Yoon S.-W., Park S.-J., Kim S.M., Choi E.-J., Si Y.-J., Lee O.-J., Shim W.-S., Kim S.-W., Mo I.-P., Bae Y., Lim Y.T., Sung M.H., Kim C.-J., Webby R.J., Webster R.G., Choi Y.K. Pathobiological features of a novel, highly pathogenic avian influenza A(H5N8) virus. Emerg. Microbes Infect. 2014;3:e75. doi: 10.1038/emi.2014.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimble J.B., Angel M., Wan H., Sutton T.C., Finch C., Perez D.R. Alternative reassortment events leading to transmissible H9N1 influenza viruses in the ferret model. J. Virol. 2014;88(1):66–71. doi: 10.1128/JVI.02677-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster F., Gouveia K., Zhou Y., Lowery K., Russell R., MacInnes H., Pollock Z., Layton R.C., Cromwell J., Toleno D., Pyle J., Zubelewicz M., Harrod K., Sampath R., Hofstadler S., Gao P., Liu Y., Cheng Y.S. Exhaled aerosol transmission of pandemic and seasonal H1N1 influenza viruses in the ferret. PLoS One. 2012;7(4):e33118. doi: 10.1371/journal.pone.0033118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreijtz J.H., Goeijenbier M., Moesker F.M., van den Dries L., Goeijenbier S., De Gruyter H.L., Lehmann M.H., Mutsert G., van de Vijver D.A., Volz A., Fouchier R.A., van Gorp E.C., Rimmelzwaan G.F., Sutter G., Osterhaus A.D. Safety and immunogenicity of a modified-vaccinia-virus-Ankara-based influenza A H5N1 vaccine: a randomised, double-blind phase 1/2a clinical trial. Lancet Infect. Dis. 2014;14(12):1196–1207. doi: 10.1016/S1473-3099(14)70963-6. [DOI] [PubMed] [Google Scholar]
- Kreijtz J.H., Kroeze E.J., Stittelaar K.J., de Waal L., van Amerongen G., van Trierum S., van Run P., Bestebroer T., Kuiken T., Fouchier R.A., Rimmelzwaan G.F., Osterhaus A.D. Low pathogenic avian influenza A(H7N9) virus causes high mortality in ferrets upon intratracheal challenge: a model to study intervention strategies. Vaccine. 2013;31(43):4995–4999. doi: 10.1016/j.vaccine.2013.06.071. [DOI] [PubMed] [Google Scholar]
- Kreijtz J.H., Suzer Y., Bodewes R., Schwantes A., van Amerongen G., Verburgh R.J., de Mutsert G., van den Brand J., van Trierum S.E., Kuiken T., Fouchier R.A., Osterhaus A.D., Sutter G., Rimmelzwaan G.F. Evaluation of a modified vaccinia virus Ankara (MVA)-based candidate pandemic influenza A/H1N1 vaccine in the ferret model. J. Gen. Virol. 2010;91(Part 11):2745–2752. doi: 10.1099/vir.0.024885-0. [DOI] [PubMed] [Google Scholar]
- Krumm S.A., Yan D., Hovingh E.S., Evers T.J., Enkirch T., Reddy G.P., Sun A., Saindane M.T., Arrendale R.F., Painter G., Liotta D.C., Natchus M.G., von Messling V., Plemper R.K. An orally available, small-molecule polymerase inhibitor shows efficacy against a lethal morbillivirus infection in a large animal model. Sci. Transl. Med. 2014;6(232) doi: 10.1126/scitranslmed.3008517. 232ra52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku K.B., Park E.H., Yum J., Kim J.A., Oh S.K., Seo S.H. Highly pathogenic avian influenza A(H5N8) virus from waterfowl, South Korea, 2014. Emerg. Infect. Dis. 2014;20(9):1587–1588. doi: 10.3201/eid2009.140390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumlin U., Olofsson S., Dimock K., Arnberg N. Sialic acid tissue distribution and influenza virus tropism. Influenza Other Respir. Viruses. 2008;2(5):147–154. doi: 10.1111/j.1750-2659.2008.00051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laddy D.J., Yan J., Kutzler M., Kobasa D., Kobinger G.P., Khan A.S., Greenhouse J., Sardesai N.Y., Draghia-Akli R., Weiner D.B. Heterosubtypic protection against pathogenic human and avian influenza viruses via in vivo electroporation of synthetic consensus DNA antigens. PLoS One. 2008;3(6):e2517. doi: 10.1371/journal.pone.0002517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert L.C., Fauci A.S. Influenza vaccines for the future. N. Engl. J. Med. 2010;363(21):2036–2044. doi: 10.1056/NEJMra1002842. [DOI] [PubMed] [Google Scholar]
- Larsen G.L., Colasurdo G.N. Neural control mechanisms within airways: disruption by respiratory syncytial virus. J. Pediatr. 1999;135(2, Part 2):21–27. [PubMed] [Google Scholar]
- Leonard V.H., Hodge G., Reyes-Del Valle J., McChesney M.B., Cattaneo R. Measles virus selectively blind to signaling lymphocytic activation molecule (SLAM; CD150) is attenuated and induces strong adaptive immune responses in rhesus monkeys. J. Virol. 2010;84(7):3413–3420. doi: 10.1128/JVI.02304-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewington J.H. 2nd ed. Elsevier Saunders; Edinburgh; New York: 2007. Ferret Husbandry, Medicine and Surgery. [Google Scholar]
- Li S., Liu C., Klimov A., Subbarao K., Perdue M.L., Mo D., Ji Y., Woods L., Hietala S., Bryant M. Recombinant influenza A virus vaccines for the pathogenic human A/Hong Kong/97 (H5N1) viruses. J. Infect. Dis. 1999;179(5):1132–1138. doi: 10.1086/314713. [DOI] [PubMed] [Google Scholar]
- Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C., Choe H., Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Sun X., Chen J., Liu X., Wisely S.M., Zhou Q., Renard J.P., Leno G.H., Engelhardt J.F. Cloned ferrets produced by somatic cell nuclear transfer. Dev. Biol. 2006;293(2):439–448. doi: 10.1016/j.ydbio.2006.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J., Zhang J., Dong X., Fang H., Chen J., Su N., Gao Q., Zhang Z., Liu Y., Wang Z., Yang M., Sun R., Li C., Lin S., Ji M., Wang X., Wood J., Feng Z., Wang Y., Yin W. Safety and immunogenicity of an inactivated adjuvanted whole-virion influenza A (H5N1) vaccine: a phase I randomised controlled trial. Lancet. 2006;368(9540):991–997. doi: 10.1016/S0140-6736(06)69294-5. [DOI] [PubMed] [Google Scholar]
- Lin S.C., Lin Y.F., Chong P., Wu S.C. Broader neutralizing antibodies against H5N1 viruses using prime-boost immunization of hyperglycosylated hemagglutinin DNA and virus-like particles. PLoS One. 2012;7(6):e39075. doi: 10.1371/journal.pone.0039075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linster M., van Boheemen S., de Graaf M., Schrauwen E.J., Lexmond P., Manz B., Bestebroer T.M., Baumann J., van Riel D., Rimmelzwaan G.F., Osterhaus A.D., Matrosovich M., Fouchier R.A., Herfst S. Identification, characterization, and natural selection of mutations driving airborne transmission of A/H5N1 virus. Cell. 2014;157(2):329–339. doi: 10.1016/j.cell.2014.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipatov A.S., Kwon Y.K., Pantin-Jackwood M.J., Swayne D.E. Pathogenesis of H5N1 influenza virus infections in mice and ferret models differs according to respiratory tract or digestive system exposure. J. Infect. Dis. 2009;199(5):717–725. doi: 10.1086/596740. [DOI] [PubMed] [Google Scholar]
- Lotz M.T., Peebles R.S., Jr. Mechanisms of respiratory syncytial virus modulation of airway immune responses. Curr. Allergy Asthma Rep. 2012;12(5):380–387. doi: 10.1007/s11882-012-0278-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby S.P., Gurley E.S., Hossain M.J. Transmission of human infection with Nipah virus. Clin. Infect. Dis. 2009;49(11):1743–1748. doi: 10.1086/647951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludlow M., Rennick L.J., Nambulli S., de Swart R.L., Duprex W.P. Using the ferret model to study morbillivirus entry, spread, transmission and cross-species infection. Curr. Opin. Virol. 2014;4:15–23. doi: 10.1016/j.coviro.2013.11.001. [DOI] [PubMed] [Google Scholar]
- MacPhail M., Schickli J.H., Tang R.S., Kaur J., Robinson C., Fouchier R.A., Osterhaus A.D., Spaete R.R., Haller A.A. Identification of small-animal and primate models for evaluation of vaccine candidates for human metapneumovirus (hMPV) and implications for hMPV vaccine design. J. Gen. Virol. 2004;85(Part 6):1655–1663. doi: 10.1099/vir.0.79805-0. [DOI] [PubMed] [Google Scholar]
- Mahmood K., Bright R.A., Mytle N., Carter D.M., Crevar C.J., Achenbach J.E., Heaton P.M., Tumpey T.M., Ross T.M. H5N1 VLP vaccine induced protection in ferrets against lethal challenge with highly pathogenic H5N1 influenza viruses. Vaccine. 2008;26(42):5393–5399. doi: 10.1016/j.vaccine.2008.07.084. [DOI] [PubMed] [Google Scholar]
- Maines T.R., Belser J.A., Gustin K.M., van Hoeven N., Zeng H., Svitek N., von Messling V., Katz J.M., Tumpey T.M. Local innate immune responses and influenza virus transmission and virulence in ferrets. J. Infect. Dis. 2012;205(3):474–485. doi: 10.1093/infdis/jir768. [DOI] [PubMed] [Google Scholar]
- Maines T.R., Chen L.M., Belser J.A., Van Hoeven N., Smith E., Donis R.O., Tumpey T.M., Katz J.M. Multiple genes contribute to the virulent phenotype observed in ferrets of an H5N1 influenza virus isolated from Thailand in 2004. Virology. 2011;413(2):226–230. doi: 10.1016/j.virol.2011.02.005. [DOI] [PubMed] [Google Scholar]
- Maines T.R., Chen L.M., Matsuoka Y., Chen H., Rowe T., Ortin J., Falcon A., Nguyen T.H., Mai le Q., Sedyaningsih E.R., Harun S., Tumpey T.M., Donis R.O., Cox N.J., Subbarao K., Katz J.M. Lack of transmission of H5N1 avian–human reassortant influenza viruses in a ferret model. Proc. Natl. Acad. Sci. USA. 2006;103(32):12121–12126. doi: 10.1073/pnas.0605134103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maines T.R., Jayaraman A., Belser J.A., Wadford D.A., Pappas C., Zeng H., Gustin K.M., Pearce M.B., Viswanathan K., Shriver Z.H., Raman R., Cox N.J., Sasisekharan R., Katz J.M., Tumpey T.M. Transmission and pathogenesis of swine-origin 2009A(H1N1) influenza viruses in ferrets and mice. Science. 2009;325(5939):484–487. doi: 10.1126/science.1177238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh G.A., Virtue E.R., Smith I., Todd S., Arkinstall R., Frazer L., Monaghan P., Smith G.A., Broder C.C., Middleton D., Wang L.F. Recombinant Hendra viruses expressing a reporter gene retain pathogenicity in ferrets. Virol. J. 2013;10:95. doi: 10.1186/1743-422X-10-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martina B.E., Haagmans B.L., Kuiken T., Fouchier R.A., Rimmelzwaan G.F., Van Amerongen G., Peiris J.S., Lim W., Osterhaus A.D. Virology: SARS virus infection of cats and ferrets. Nature. 2003;425(6961):915. doi: 10.1038/425915a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascoli C.C., Gower T.A., Capilupo F.A., Metzgar D.P. Further studies on the neonatal ferret model of infection and immunity to and attenuation of human parainfluenza viruses. Dev. Biol. Stand. 1976;33:384–390. [PubMed] [Google Scholar]
- Mascoli C.C., Metzgar D.P., Larson E.J., Fuscaldo A.A., Gower T.A. An animal model for studying infection and immunity to and attenuation of human parainfluenza viruses. Dev. Biol. Stand. 1975;28:414–421. [PubMed] [Google Scholar]
- Matrosovich M., Tuzikov A., Bovin N., Gambaryan A., Klimov A., Castrucci M.R., Donatelli I., Kawaoka Y. Early alterations of the receptor-binding properties of H1, H2, and H3 avian influenza virus hemagglutinins after their introduction into mammals. J. Virol. 2000;74(18):8502–8512. doi: 10.1128/jvi.74.18.8502-8512.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullers J.A., McAuley J.L., Browall S., Iverson A.R., Boyd K.L., Henriques Normark B. Influenza enhances susceptibility to natural acquisition of and disease due to Streptococcus pneumoniae in ferrets. J. Infect. Dis. 2010;202(8):1287–1295. doi: 10.1086/656333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier I., Embury-Hyatt C., Stebner S., Gray M., Bastien N., Li Y., Plummer F., Kobinger G.P., von Messling V. Virulence differences of closely related pandemic 2009 H1N1 isolates correlate with increased inflammatory responses in ferrets. Virology. 2012;422(1):125–131. doi: 10.1016/j.virol.2011.10.018. [DOI] [PubMed] [Google Scholar]
- Middleton D., Pallister J., Klein R., Feng Y.R., Haining J., Arkinstall R., Frazer L., Huang J.A., Edwards N., Wareing M., Elhay M., Hashmi Z., Bingham J., Yamada M., Johnson D., White J., Foord A., Heine H.G., Marsh G.A., Broder C.C., Wang L.F. Hendra virus vaccine, a one health approach to protecting horse, human, and environmental health. Emerg. Infect. Dis. 2014;20(3):372–379. doi: 10.3201/eid2003.131159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mire C.E., Versteeg K.M., Cross R.W., Agans K.N., Fenton K.A., Whitt M.A., Geisbert T.W. Single injection recombinant vesicular stomatitis virus vaccines protect ferrets against lethal Nipah virus disease. Virol. J. 2013;10:353. doi: 10.1186/1743-422X-10-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore I.N., Lamirande E.W., Paskel M., Donahue D., Qin J., Subbarao K. Severity of clinical disease and pathology in ferrets experimentally infected with influenza viruses is influenced by inoculum volume. J. Virol. 2014;88(23):13879–13891. doi: 10.1128/JVI.02341-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlebach M.D., Mateo M., Sinn P.L., Prufer S., Uhlig K.M., Leonard V.H., Navaratnarajah C.K., Frenzke M., Wong X.X., Sawatsky B., Ramachandran S., McCray P.B., Jr., Cichutek K., von Messling V., Lopez M., Cattaneo R. Adherens junction protein nectin-4 is the epithelial receptor for measles virus. Nature. 2011;480(7378):530–533. doi: 10.1038/nature10639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munster V.J., de Wit E., van den Brand J.M., Herfst S., Schrauwen E.J., Bestebroer T.M., van de Vijver D., Boucher C.A., Koopmans M., Rimmelzwaan G.F., Kuiken T., Osterhaus A.D., Fouchier R.A. Pathogenesis and transmission of swine-origin 2009A(H1N1) influenza virus in ferrets. Science. 2009;325(5939):481–483. doi: 10.1126/science.1177127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata T., Lefor A.K., Hasegawa M., Ishii M. Favipiravir: a new medication for the ebola virus disease pandemic. Disaster Med. Public Health Prep. 2014:1–3. doi: 10.1017/dmp.2014.151. [DOI] [PubMed] [Google Scholar]
- Nair H., Nokes D.J., Gessner B.D., Dherani M., Madhi S.A., Singleton R.J., O׳Brien K.L., Roca A., Wright P.F., Bruce N., Chandran A., Theodoratou E., Sutanto A., Sedyaningsih E.R., Ngama M., Munywoki P.K., Kartasasmita C., Simoes E.A., Rudan I., Weber M.W., Campbell H. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010;375(9725):1545–1555. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen H.T., Fry A.M., Gubareva L.V. Neuraminidase inhibitor resistance in influenza viruses and laboratory testing methods. Antivir. Ther. 2012;17(1, Part B):159–173. doi: 10.3851/IMP2067. [DOI] [PubMed] [Google Scholar]
- Nicholls J.M., Chan R.W., Russell R.J., Air G.M., Peiris J.S. Evolving complexities of influenza virus and its receptors. Trends Microbiol. 2008;16(4):149–157. doi: 10.1016/j.tim.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Niezgoda M., Briggs D.J., Shaddock J., Dreesen D.W., Rupprecht C.E. Pathogenesis of experimentally induced rabies in domestic ferrets. Am. J. Vet. Res. 1997;58(11):1327–1331. [PubMed] [Google Scholar]
- Noyce R.S., Bondre D.G., Ha M.N., Lin L.T., Sisson G., Tsao M.S., Richardson C.D. Tumor cell marker PVRL4 (nectin 4) is an epithelial cell receptor for measles virus. PLoS Pathog. 2011;7(8):e1002240. doi: 10.1371/journal.ppat.1002240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O׳Sullivan J.D., Allworth A.M., Paterson D.L., Snow T.M., Boots R., Gleeson L.J., Gould A.R., Hyatt A.D., Bradfield J. Fatal encephalitis due to novel paramyxovirus transmitted from horses. Lancet. 1997;349(9045):93–95. doi: 10.1016/s0140-6736(96)06162-4. [DOI] [PubMed] [Google Scholar]
- Olson J.G., Rupprecht C., Rollin P.E., An U.S., Niezgoda M., Clemins T., Walston J., Ksiazek T.G. Antibodies to Nipah-like virus in bats (Pteropus lylei), Cambodia. Emerg. Infect. Dis. 2002;8(9):987–988. doi: 10.3201/eid0809.010515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishi M., Kitano M., Taniguchi K., Homma T., Kobayashi M., Sato A., Coban C., Ishii K.J. Hemozoin is a potent adjuvant for hemagglutinin split vaccine without pyrogenicity in ferrets. Vaccine. 2014;32(25):3004–3009. doi: 10.1016/j.vaccine.2014.03.072. [DOI] [PubMed] [Google Scholar]
- Palese P., Shaw ML. Knipe D.M., Howley P.M. (Eds.), Fields Virology. 5th edition. Lippincott Williams; Philadelphia: 2007. Orthomyxoviridae: the viruses and their replication. [Google Scholar]
- Pallister J., Middleton D., Crameri G., Yamada M., Klein R., Hancock T.J., Foord A., Shiell B., Michalski W., Broder C.C., Wang L.F. Chloroquine administration does not prevent Nipah virus infection and disease in ferrets. J. Virol. 2009;83(22):11979–11982. doi: 10.1128/JVI.01847-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallister J., Middleton D., Wang L.F., Klein R., Haining J., Robinson R., Yamada M., White J., Payne J., Feng Y.R., Chan Y.P., Broder C.C. A recombinant Hendra virus G glycoprotein-based subunit vaccine protects ferrets from lethal Hendra virus challenge. Vaccine. 2011;29(34):5623–5630. doi: 10.1016/j.vaccine.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallister J.A., Klein R., Arkinstall R., Haining J., Long F., White J.R., Payne J., Feng Y.R., Wang L.F., Broder C.C., Middleton D. Vaccination of ferrets with a recombinant G glycoprotein subunit vaccine provides protection against Nipah virus disease for over 12 months. Virol. J. 2013;10:237. doi: 10.1186/1743-422X-10-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker L., Gilliland S.M., Minor P., Schepelmann S. Assessment of the ferret as an in vivo model for mumps virus infection. J. Gen. Virol. 2013;94(Part 6):1200–1205. doi: 10.1099/vir.0.052449-0. [DOI] [PubMed] [Google Scholar]
- Peiris J.S., Cheung C.Y., Leung C.Y., Nicholls J.M. Innate immune responses to influenza A H5N1: friend or foe? Trends Immunol. 2009;30(12):574–584. doi: 10.1016/j.it.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris J.S., Chu C.M., Cheng V.C., Chan K.S., Hung I.F., Poon L.L., Law K.I., Tang B.S., Hon T.Y., Chan C.S., Chan K.H., Ng J.S., Zheng B.J., Ng W.L., Lai R.W., Guan Y., Yuen K.Y. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361(9371):1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltola V.T., Boyd K.L., McAuley J.L., Rehg J.E., McCullers J.A. Bacterial sinusitis and otitis media following influenza virus infection in ferrets. Infect. Immun. 2006;74(5):2562–2567. doi: 10.1128/IAI.74.5.2562-2567.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X., Alfoldi J., Gori K., Eisfeld A.J., Tyler S.R., Tisoncik-Go J., Brawand D., Law G.L., Skunca N., Hatta M., Gasper D.J., Kelly S.M., Chang J., Thomas M.J., Johnson J., Berlin A.M., Lara M., Russell P., Swofford R., Turner-Maier J., Young S., Hourlier T., Aken B., Searle S., Sun X., Yi Y., Suresh M., Tumpey T.M., Siepel A., Wisely S.M., Dessimoz C., Kawaoka Y., Birren B.W., Lindblad-Toh K., Di Palma F., Engelhardt J.F., Palermo R.E., Katze M.G. The draft genome sequence of the ferret (Mustela putorius furo) facilitates study of human respiratory disease. Nat. Biotechnol. 2014;32(12):1250–1255. doi: 10.1038/nbt.3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petsch B., Schnee M., Vogel A.B., Lange E., Hoffmann B., Voss D., Schlake T., Thess A., Kallen K.J., Stitz L., Kramps T. Protective efficacy of in vitro synthesized, specific mRNA vaccines against influenza A virus infection. Nat. Biotechnol. 2012;30(12):1210–1216. doi: 10.1038/nbt.2436. [DOI] [PubMed] [Google Scholar]
- Pillet S., Kobasa D., Meunier I., Gray M., Laddy D., Weiner D.B., von Messling V., Kobinger G.P. Cellular immune response in the presence of protective antibody levels correlates with protection against 1918 influenza in ferrets. Vaccine. 2011;29(39):6793–6801. doi: 10.1016/j.vaccine.2010.12.059. [DOI] [PubMed] [Google Scholar]
- Pillet S., von Messling V. Canine distemper virus selectively inhibits apoptosis progression in infected immune cells. J. Virol. 2009;83(12):6279–6287. doi: 10.1128/JVI.00050-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Playford E.G., McCall B., Smith G., Slinko V., Allen G., Smith I., Moore F., Taylor C., Kung Y.H., Field H. Human Hendra virus encephalitis associated with equine outbreak, Australia, 2008. Emerg. Infect. Dis. 2010;16(2):219–223. doi: 10.3201/eid1602.090552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poland G.A., Sambhara S. Vaccines against influenza A (H5N1): evidence of progress. J. Infect. Dis. 2008;198(5):629–631. doi: 10.1086/590912. [DOI] [PubMed] [Google Scholar]
- Prince G.A., Porter D.D. The pathogenesis of respiratory syncytial virus infection in infant ferrets. Am. J. Pathol. 1976;82(2):339–352. [PMC free article] [PubMed] [Google Scholar]
- Pushko P., Pearce M.B., Ahmad A., Tretyakova I., Smith G., Belser J.A., Tumpey T.M. Influenza virus-like particle can accommodate multiple subtypes of hemagglutinin and protect from multiple influenza types and subtypes. Vaccine. 2011;29(35):5911–5918. doi: 10.1016/j.vaccine.2011.06.068. [DOI] [PubMed] [Google Scholar]
- Raj V.S., Mou H., Smits S.L., Dekkers D.H., Muller M.A., Dijkman R., Muth D., Demmers J.A., Zaki A., Fouchier R.A., Thiel V., Drosten C., Rottier P.J., Osterhaus A.D., Bosch B.J., Haagmans B.L. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature. 2013;495(7440):251–254. doi: 10.1038/nature12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran A., Parisien J.P., Horvath C.M. STAT2 is a primary target for measles virus V protein-mediated alpha/beta interferon signaling inhibition. J. Virol. 2008;82(17):8330–8338. doi: 10.1128/JVI.00831-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S.S., Kong W.P., Wei C.J., Van Hoeven N., Gorres J.P., Nason M., Andersen H., Tumpey T.M., Nabel G.J. Comparative efficacy of hemagglutinin, nucleoprotein, and matrix 2 protein gene-based vaccination against H5N1 influenza in mouse and ferret. PLoS One. 2010;5(3):e9812. doi: 10.1371/journal.pone.0009812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard M., Schrauwen E.J., de Graaf M., Bestebroer T.M., Spronken M.I., van Boheemen S., de Meulder D., Lexmond P., Linster M., Herfst S., Smith D.J., van den Brand J.M., Burke D.F., Kuiken T., Rimmelzwaan G.F., Osterhaus A.D., Fouchier R.A. Limited airborne transmission of H7N9 influenza A virus between ferrets. Nature. 2013;501(7468):560–563. doi: 10.1038/nature12476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimmelzwaan G.F., Sutter G. Candidate influenza vaccines based on recombinant modified vaccinia virus Ankara. Expert Rev. Vaccines. 2009;8(4):447–454. doi: 10.1586/erv.09.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson N.P., Venning L., Kyle H., Widdicombe J.G. Quantitation of the secretory cells of the ferret tracheobronchial tree. J. Anat. 1986;145:173–188. [PMC free article] [PubMed] [Google Scholar]
- Rodgers L., Pabst L.J., Chaves S.S. Increasing uptake of live attenuated influenza vaccine among children in the United States, 2008–2014. Vaccine. 2015;33(1):22–24. doi: 10.1016/j.vaccine.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorke L.B., Fabiyi A., Elizan T.S., Sever J.L. Experimental cerebrovascular lesions in congenital and neonatal rubella-virus infections of ferrets. Lancet. 1968;2(7560):153–154. doi: 10.1016/s0140-6736(68)90428-5. [DOI] [PubMed] [Google Scholar]
- Ross T.M., Mahmood K., Crevar C.J., Schneider-Ohrum K., Heaton P.M., Bright R.A. A trivalent virus-like particle vaccine elicits protective immune responses against seasonal influenza strains in mice and ferrets. PLoS One. 2009;4(6):e6032. doi: 10.1371/journal.pone.0006032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothlisberger A., Wiener D., Schweizer M., Peterhans E., Zurbriggen A., Plattet P. Two domains of the V protein of virulent canine distemper virus selectively inhibit STAT1 and STAT2 nuclear import. J. Virol. 2010;84(13):6328–6343. doi: 10.1128/JVI.01878-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouxel R.N., Svitek N., von Messling V. A chimeric measles virus with canine distemper envelope protects ferrets from lethal distemper challenge. Vaccine. 2009;27(36):4961–4966. doi: 10.1016/j.vaccine.2009.05.096. [DOI] [PubMed] [Google Scholar]
- Rowe T., Leon A.J., Crevar C.J., Carter D.M., Xu L., Ran L., Fang Y., Cameron C.M., Cameron M.J., Banner D., Ng D.C., Ran R., Weirback H.K., Wiley C.A., Kelvin D.J., Ross T.M. Modeling host responses in ferrets during A/California/07/2009 influenza infection. Virology. 2010;401(2):257–265. doi: 10.1016/j.virol.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd P.A., Cattaneo R., von Messling V. Canine distemper virus uses both the anterograde and the hematogenous pathway for neuroinvasion. J. Virol. 2006;80(19):9361–9370. doi: 10.1128/JVI.01034-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupprecht C.E., Gilbert J., Pitts R., Marshall K.R., Koprowski H. Evaluation of an inactivated rabies virus vaccine in domestic ferrets. J. Am. Vet. Med. Assoc. 1990;196(10):1614–1616. [PubMed] [Google Scholar]
- Rutigliano J.A., Doherty P.C., Franks J., Morris M.Y., Reynolds C., Thomas P.G. Screening monoclonal antibodies for cross-reactivity in the ferret model of influenza infection. J. Immunol. Methods. 2008;336(1):71–77. doi: 10.1016/j.jim.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawatsky B., Wong X.X., Hinkelmann S., Cattaneo R., von Messling V. Canine distemper virus epithelial cell infection is required for clinical disease but not for immunosuppression. J. Virol. 2012;86(7):3658–3666. doi: 10.1128/JVI.06414-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See R.H., Petric M., Lawrence D.J., Mok C.P., Rowe T., Zitzow L.A., Karunakaran K.P., Voss T.G., Brunham R.C., Gauldie J., Finlay B.B., Roper R.L. Severe acute respiratory syndrome vaccine efficacy in ferrets: whole killed virus and adenovirus-vectored vaccines. J. Gen. Virol. 2008;89(Pt 9):2136–2146. doi: 10.1099/vir.0.2008/001891-0. [DOI] [PubMed] [Google Scholar]
- Sherrill A., Gorham J. Bone marrow hypoplasia associated with estrus in ferrets. Lab. Anim. Sci. 1985;35(3):280–286. [PubMed] [Google Scholar]
- Shinya K., Ebina M., Yamada S., Ono M., Kasai N., Kawaoka Y. Avian flu: influenza virus receptors in the human airway. Nature. 2006;440(7083):435–436. doi: 10.1038/440435a. [DOI] [PubMed] [Google Scholar]
- Shinya K., Hamm S., Hatta M., Ito H., Ito T., Kawaoka Y. PB2 amino acid at position 627 affects replicative efficiency, but not cell tropism, of Hong Kong H5N1 influenza A viruses in mice. Virology. 2004;320(2):258–266. doi: 10.1016/j.virol.2003.11.030. [DOI] [PubMed] [Google Scholar]
- Smith H., Sweet C. Lessons for human influenza from pathogenicity studies with ferrets. Rev. Infect. Dis. 1988;10(1):56–75. doi: 10.1093/clinids/10.1.56. [DOI] [PubMed] [Google Scholar]
- Smith W., Andrewes C.H., Laidlaw P.P. A virus obtained from influenza patients. Lancet. 1933;222:66. [Google Scholar]
- Stevens J., Blixt O., Chen L.M., Donis R.O., Paulson J.C., Wilson I.A. Recent avian H5N1 viruses exhibit increased propensity for acquiring human receptor specificity. J. Mol. Biol. 2008;381(5):1382–1394. doi: 10.1016/j.jmb.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbarao K., Luke C. H5N1 viruses and vaccines. PLoS Pathog. 2007;3(3):e40. doi: 10.1371/journal.ppat.0030040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suguitan A.L., Jr., Cheng X., Wang W., Wang S., Jin H., Lu S. Influenza H5 hemagglutinin DNA primes the antibody response elicited by the live attenuated influenza A/Vietnam/1203/2004 vaccine in ferrets. PLoS One. 2011;6(7):e21942. doi: 10.1371/journal.pone.0021942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suguitan A.L., Jr., McAuliffe J., Mills K.L., Jin H., Duke G., Lu B., Luke C.J., Murphy B., Swayne D.E., Kemble G., Subbarao K. Live, attenuated influenza A H5N1 candidate vaccines provide broad cross-protection in mice and ferrets. PLoS Med. 2006;3(9):e360. doi: 10.1371/journal.pmed.0030360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X., Sui H., Fisher J.T., Yan Z., Liu X., Cho H.J., Joo N.S., Zhang Y., Zhou W., Yi Y., Kinyon J.M., Lei-Butters D.C., Griffin M.A., Naumann P., Luo M., Ascher J., Wang K., Frana T., Wine J.J., Meyerholz D.K., Engelhardt J.F. Disease phenotype of a ferret CFTR-knockout model of cystic fibrosis. J. Clin. Invest. 2010;120(9):3149–3160. doi: 10.1172/JCI43052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton T.C., Finch C., Shao H., Angel M., Chen H., Capua I., Cattoli G., Monne I., Perez D.R. Airborne transmission of highly pathogenic H7N1 influenza virus in ferrets. J. Virol. 2014;88(12):6623–6635. doi: 10.1128/JVI.02765-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svitek N., Gerhauser I., Goncalves C., Grabski E., Doring M., Kalinke U., Anderson D.E., Cattaneo R., von Messling V. Morbillivirus control of the interferon response: relevance of STAT2 and mda5 but not STAT1 for canine distemper virus virulence in ferrets. J. Virol. 2014;88(5):2941–2950. doi: 10.1128/JVI.03076-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svitek N., Rudd P.A., Obojes K., Pillet S., von Messling V. Severe seasonal influenza in ferrets correlates with reduced interferon and increased IL-6 induction. Virology. 2008;376(1):53–59. doi: 10.1016/j.virol.2008.02.035. [DOI] [PubMed] [Google Scholar]
- Svitek N., von Messling V. Early cytokine mRNA expression profiles predict Morbillivirus disease outcome in ferrets. Virology. 2007;362(2):404–410. doi: 10.1016/j.virol.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet C., Hayden F.G., Jakeman K.J., Grambas S., Hay A.J. Virulence of rimantadine-resistant human influenza A (H3N2) viruses in ferrets. J. Infect. Dis. 1991;164(5):969–972. doi: 10.1093/infdis/164.5.969. [DOI] [PubMed] [Google Scholar]
- Tan C.T., Goh K.J., Wong K.T., Sarji S.A., Chua K.B., Chew N.K., Murugasu P., Loh Y.L., Chong H.T., Tan K.S., Thayaparan T., Kumar S., Jusoh M.R. Relapsed and late-onset Nipah encephalitis. Ann. Neurol. 2002;51(6):703–708. doi: 10.1002/ana.10212. [DOI] [PubMed] [Google Scholar]
- Tang L., González R., Dobrinski I. Germline modification of domestic animals. Anim. Reprod. 2015;12(1):93–104. [PMC free article] [PubMed] [Google Scholar]
- Tatsuo H., Ono N., Tanaka K., Yanagi Y. SLAM (CDw150) is a cellular receptor for measles virus. Nature. 2000;406(6798):893–897. doi: 10.1038/35022579. [DOI] [PubMed] [Google Scholar]
- Tatsuo H., Ono N., Yanagi Y. Morbilliviruses use signaling lymphocyte activation molecules (CD150) as cellular receptors. J. Virol. 2001;75(13):5842–5850. doi: 10.1128/JVI.75.13.5842-5850.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubenberger J.K., Reid A.H., Krafft A.E., Bijwaard K.E., Fanning T.G. Initial genetic characterization of the 1918 “Spanish” influenza virus. Science. 1997;275(5307):1793–1796. doi: 10.1126/science.275.5307.1793. [DOI] [PubMed] [Google Scholar]
- ter Meulen J., Bakker A.B., van den Brink E.N., Weverling G.J., Martina B.E., Haagmans B.L., Kuiken T., de Kruif J., Preiser W., Spaan W., Gelderblom H.R., Goudsmit J., Osterhaus A.D. Human monoclonal antibody as prophylaxis for SARS coronavirus infection in ferrets. Lancet. 2004;363(9427):2139–2141. doi: 10.1016/S0140-6736(04)16506-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To K.F., Chan P.K., Chan K.F., Lee W.K., Lam W.Y., Wong K.F., Tang N.L., Tsang D.N., Sung R.Y., Buckley T.A., Tam J.S., Cheng A.F. Pathology of fatal human infection associated with avian influenza A H5N1 virus. J. Med. Virol. 2001;63(3):242–246. doi: 10.1002/1096-9071(200103)63:3<242::aid-jmv1007>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Tong S., Li Y., Rivailler P., Conrardy C., Castillo D.A., Chen L.M., Recuenco S., Ellison J.A., Davis C.T., York I.A., Turmelle A.S., Moran D., Rogers S., Shi M., Tao Y., Weil M.R., Tang K., Rowe L.A., Sammons S., Xu X., Frace M., Lindblade K.A., Cox N.J., Anderson L.J., Rupprecht C.E., Donis R.O. A distinct lineage of influenza A virus from bats. Proc. Natl. Acad. Sci. USA. 2012;109(11):4269–4274. doi: 10.1073/pnas.1116200109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong S., Zhu X., Li Y., Shi M., Zhang J., Bourgeois M., Yang H., Chen X., Recuenco S., Gomez J., Chen L.M., Johnson A., Tao Y., Dreyfus C., Yu W., McBride R., Carney P.J., Gilbert A.T., Chang J., Guo Z., Davis C.T., Paulson J.C., Stevens J., Rupprecht C.E., Holmes E.C., Wilson I.A., Donis R.O. New world bats harbor diverse influenza A viruses. PLoS Pathog. 2013;9(10):e1003657. doi: 10.1371/journal.ppat.1003657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretyakova I., Pearce M.B., Florese R., Tumpey T.M., Pushko P. Intranasal vaccination with H5, H7 and H9 hemagglutinins co-localized in a virus-like particle protects ferrets from multiple avian influenza viruses. Virology. 2013;442(1):67–73. doi: 10.1016/j.virol.2013.03.027. [DOI] [PubMed] [Google Scholar]
- Tumpey T.M., Basler C.F., Aguilar P.V., Zeng H., Solorzano A., Swayne D.E., Cox N.J., Katz J.M., Taubenberger J.K., Palese P., Garcia-Sastre A. Characterization of the reconstructed 1918 Spanish influenza pandemic virus. Science. 2005;310(5745):77–80. doi: 10.1126/science.1119392. [DOI] [PubMed] [Google Scholar]
- Tumpey T.M., Maines T.R., Van Hoeven N., Glaser L., Solorzano A., Pappas C., Cox N.J., Swayne D.E., Palese P., Katz J.M., Garcia-Sastre A. A two-amino acid change in the hemagglutinin of the 1918 influenza virus abolishes transmission. Science. 2007;315(5812):655–659. doi: 10.1126/science.1136212. [DOI] [PubMed] [Google Scholar]
- van den Brand J.M., Kreijtz J.H., Bodewes R., Stittelaar K.J., van Amerongen G., Kuiken T., Simon J., Fouchier R.A., Del Giudice G., Rappuoli R., Rimmelzwaan G.F., Osterhaus A.D. Efficacy of vaccination with different combinations of MF59-adjuvanted and nonadjuvanted seasonal and pandemic influenza vaccines against pandemic H1N1 (2009) influenza virus infection in ferrets. J. Virol. 2011;85(6):2851–2858. doi: 10.1128/JVI.01939-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Brand J.M., Stittelaar K.J., van Amerongen G., Reperant L., de Waal L., Osterhaus A.D., Kuiken T. Comparison of temporal and spatial dynamics of seasonal H3N2, pandemic H1N1 and highly pathogenic avian influenza H5N1 virus infections in ferrets. PLoS One. 2012;7(8):e42343. doi: 10.1371/journal.pone.0042343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Hoogen B.G., de Jong J.C., Groen J., Kuiken T., de Groot R., Fouchier R.A., Osterhaus A.D. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat. Med. 2001;7(6):719–724. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Hoogen B.G., van Doornum G.J., Fockens J.C., Cornelissen J.J., Beyer W.E., de Groot R., Osterhaus A.D., Fouchier R.A. Prevalence and clinical symptoms of human metapneumovirus infection in hospitalized patients. J. Infect. Dis. 2003;188(10):1571–1577. doi: 10.1086/379200. [DOI] [PubMed] [Google Scholar]
- van Doremalen N., Miazgowicz K.L., Milne-Price S., Bushmaker T., Robertson S., Scott D., Kinne J., McLellan J.S., Zhu J., Munster V.J. Host species restriction of Middle East respiratory syndrome coronavirus through its receptor, dipeptidyl peptidase 4. J. Virol. 2014;88(16):9220–9232. doi: 10.1128/JVI.00676-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Riel D., Munster V.J., de Wit E., Rimmelzwaan G.F., Fouchier R.A., Osterhaus A.D., Kuiken T. Human and avian influenza viruses target different cells in the lower respiratory tract of humans and other mammals. Am. J. Pathol. 2007;171(4):1215–1223. doi: 10.2353/ajpath.2007.070248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Messling V., Oezguen N., Zheng Q., Vongpunsawad S., Braun W., Cattaneo R. Nearby clusters of hemagglutinin residues sustain SLAM-dependent canine distemper virus entry in peripheral blood mononuclear cells. J. Virol. 2005;79(9):5857–5862. doi: 10.1128/JVI.79.9.5857-5862.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Messling V., Springfeld C., Devaux P., Cattaneo R. A ferret model of canine distemper virus virulence and immunosuppression. J. Virol. 2003;77(23):12579–12591. doi: 10.1128/JVI.77.23.12579-12591.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos A., Muller T., Cox J., Neubert L., Fooks A.R. Susceptibility of ferrets (Mustela putorius furo) to experimentally induced rabies with European Bat Lyssaviruses (EBLV) J. Vet Med. B Infect. Dis. Vet Public Health. 2004;51(2):55–60. doi: 10.1111/j.1439-0450.2004.00730.x. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Kiso M., Fukuyama S., Nakajima N., Imai M., Yamada S., Murakami S., Yamayoshi S., Iwatsuki-Horimoto K., Sakoda Y., Takashita E., McBride R., Noda T., Hatta M., Imai H., Zhao D., Kishida N., Shirakura M., de Vries R.P., Shichinohe S., Okamatsu M., Tamura T., Tomita Y., Fujimoto N., Goto K., Katsura H., Kawakami E., Ishikawa I., Watanabe S., Ito M., Sakai-Tagawa Y., Sugita Y., Uraki R., Yamaji R., Eisfeld A.J., Zhong G., Fan S., Ping J., Maher E.A., Hanson A., Uchida Y., Saito T., Ozawa M., Neumann G., Kida H., Odagiri T., Paulson J.C., Hasegawa H., Tashiro M., Kawaoka Y. Characterization of H7N9 influenza A viruses isolated from humans. Nature. 2013;501(7468):551–555. doi: 10.1038/nature12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingartl H., Czub M., Czub S., Neufeld J., Marszal P., Gren J., Smith G., Jones S., Proulx R., Deschambault Y., Grudeski E., Andonov A., He R., Li Y., Copps J., Grolla A., Dick D., Berry J., Ganske S., Manning L., Cao J. Immunization with modified vaccinia virus Ankara-based recombinant vaccine against severe acute respiratory syndrome is associated with enhanced hepatitis in ferrets. J. Virol. 2004;78(22):12672–12676. doi: 10.1128/JVI.78.22.12672-12676.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welter J., Taylor J., Tartaglia J., Paoletti E., Stephensen C.B. Vaccination against canine distemper virus infection in infant ferrets with and without maternal antibody protection, using recombinant attenuated poxvirus vaccines. J. Virol. 2000;74(14):6358–6367. doi: 10.1128/jvi.74.14.6358-6367.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K.T., Robertson T., Ong B.B., Chong J.W., Yaiw K.C., Wang L.F., Ansford A.J., Tannenberg A. Human Hendra virus infection causes acute and relapsing encephalitis. Neuropathol. Appl. Neurobiol. 2009;35(3):296–305. doi: 10.1111/j.1365-2990.2008.00991.x. [DOI] [PubMed] [Google Scholar]
- Wu H., Peng X., Xu L., Jin C., Cheng L., Lu X., Xie T., Yao H., Wu N. Novel reassortant influenza A(H5N8) viruses in domestic ducks, eastern China. Emerg. Infect. Dis. 2014;20(8):1315–1318. doi: 10.3201/eid2008.140339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P., Huang Z., Gao X., Michel F.J., Hirsch G., Hogan R.J., Sakamoto K., Ho W., Wu J., He B. Infection of mice, ferrets, and rhesus macaques with a clinical mumps virus isolate. J. Virol. 2013;87(14):8158–8168. doi: 10.1128/JVI.01028-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q., Chen Z., Cheng X., Xu L., Jin H. Evaluation of live attenuated H7N3 and H7N7 vaccine viruses for their receptor binding preferences, immunogenicity in ferrets and cross reactivity to the novel H7N9 virus. PLoS One. 2013;8(10):e76884. doi: 10.1371/journal.pone.0076884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yager E.J., Stagnar C., Gopalakrishnan R., Fuller J.T., Fuller D.H. Optimizing particle-mediated epidermal delivery of an influenza DNA vaccine in ferrets. Methods Mol. Biol. 2013;940:223–237. doi: 10.1007/978-1-62703-110-3_19. [DOI] [PubMed] [Google Scholar]
- Yen H.L., Lipatov A.S., Ilyushina N.A., Govorkova E.A., Franks J., Yilmaz N., Douglas A., Hay A., Krauss S., Rehg J.E., Hoffmann E., Webster R.G. Inefficient transmission of H5N1 influenza viruses in a ferret contact model. J. Virol. 2007;81(13):6890–6898. doi: 10.1128/JVI.00170-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young P.L., Halpin K., Selleck P.W., Field H., Gravel J.L., Kelly M.A., Mackenzie J.S. Serologic evidence for the presence in Pteropus bats of a paramyxovirus related to equine morbillivirus. Emerg. Infect. Dis. 1996;2(3):239–240. doi: 10.3201/eid0203.960315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D., Fouchier R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012;367(19):1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- Zamoto A., Taguchi F., Fukushi S., Morikawa S., Yamada Y.K. Identification of ferret ACE2 and its receptor function for SARS-coronavirus. Adv. Exp. Med. Biol. 2006;581:519–522. doi: 10.1007/978-0-387-33012-9_93. [DOI] [PMC free article] [PubMed] [Google Scholar]