SUMMARY
Background
Avian influenza A(H7N9) virus has caused human infections in China since 2013, and a large epidemic in 2016–17 has prompted concerns of whether the epidemiology has changed to suggest an increasing pandemic threat. Our study aimed to describe the epidemiological characteristics, clinical severity, and time-to-event distributions of A(H7N9) case-patients in the 2016–17 epidemic wave compared with previous waves.
Methods
We obtained information about all laboratory-confirmed human cases of A(H7N9) virus infection reported in mainland China as of 23 February 2017. We described the epidemiological characteristics across epidemic waves, and estimated the risk for death, mechanical ventilation, and admission to the intensive care unit for patients who required hospitalization for medical reasons. We estimated the incubation periods, and time delays from illness onset to hospital admission, illness onset to initiation of antiviral treatment, and hospital admission to death or discharge.
Findings
The 2016–17 A(H7N9) epidemic wave began earlier, spread to more counties in affected provinces and had more confirmed cases than previous epidemic waves. There was an increase in the proportion of cases in middle-aged adults and in semi-urban and rural residents. The clinical severity of hospitalized cases in 2016–17 was comparable to the previous epidemic waves.
Interpretation
Age distribution and case sources changed gradually across epidemic waves, while clinical severity has not changed substantially. Continued vigilance and sustained intensive control efforts are needed to minimize the risk of human infection with A(H7N9) virus.
Funding
The National Science Fund for Distinguished Young Scholars (grant no. 81525023).
Keywords: Avian influenza, H7N9, Epidemiology, Risk assessment
INTRODUCTION
The emergence of novel avian influenza A(H7N9) virus in 2013 poses a pandemic threat to humans, which necessitates close monitoring of the virus and continuing assessment of pandemic risk.1 Human cases of A(H7N9) virus infection have occurred during annual winter-spring epidemic waves in mainland China since 2013,1 reaching 1,220 laboratory-confirmed cases and 494 deaths as of 23 February 2017. After peaking during 2013–14, the number of cases identified in subsequent epidemic waves was generally lower, until a surge in A(H7N9) cases was observed in December 2016, in the 5th epidemic wave of human infections.2 The 5th epidemic wave that started on 1 October 2016 has included 447 laboratory-confirmed cases in mainland China (as of 23 February 2017), and is the largest epidemic wave to date. The earlier start and larger epidemic size in the 5th A(H7N9) epidemic wave has prompted concerns of whether the epidemiology has changed to suggest an increasing pandemic threat.2,3
Prior to 2016–17, the avian influenza A(H7N9) viruses circulating among poultry in China have been classified as low pathogenic, and infected poultry have been asymptomatic. Experimental studies have reported that some A(H7N9) virus strains have acquired tropism to bind to receptors that are present in the human upper respiratory tract that could facilitate increased transmissibility among humans.4,5 On 19 February 2017, the Chinese Center for Disease Control and Prevention (China CDC) reported that A(H7N9) virus with a four basic amino-acid insertion in a host protease cleavage site in the HA protein was identified in two patients with A(H7N9) virus infection in Guangdong province,6 suggesting that some A(H7N9) virus strains have evolved to become highly pathogenic in poultry.
A(H7N9) virus has the highest risk score among the 12 novel influenza A viruses evaluated by the Influenza Risk Assessment Tool to date, and is characterized as posing moderate-high potential pandemic risk by the U.S. Centers for Disease Control and Prevention.3 In addition to the on-going monitoring of virological and molecular characteristics of A(H7N9) viruses recovered from infected poultry and humans, continuous epidemiological investigations and clinical severity assessments of A(H7N9) case-patients are critical to inform public health for pandemic preparedness.7–12 The present study aims to assess changes of basic epidemiology, clinical severity and key epidemiological parameters of time-to-event distributions of A(H7N9) case-patients in the 5th epidemic wave compared with previous epidemic waves in mainland China.
METHODS
Data
We collected individual records of all laboratory-confirmed human cases of avian influenza A(H7N9) virus infection in mainland China from an integrated electronic database managed by China CDC and provincial CDCs. Every identified human case of A(H7N9) virus infection was required to be reported to China CDC within 24 hours via a national surveillance system for notifiable infectious diseases. Staff from the local CDCs were responsible for field investigations to collect data using a standardized form. Case definitions, exposure definitions, surveillance for identification of cases, and laboratory testing for A(H7N9) virus have been described in detail elsewhere.7,13,14 We extracted data about patients’ demographic, epidemiological and clinical characteristics, time-to-event dates, and health outcomes on 23 February 2017. Details about the extracted variables are described in the Appendix. We defined the 1st epidemic wave from 1 January 2013 to 30 September 2013, and subsequent epidemic waves from October 1 to September 30 of the following year.
Statistical analysis
In clinical severity analysis, we selected A(H7N9) case-patients who required hospitalization because of serious illness, thus excluding those clinically mild cases admitted into hospitals only for isolation purposes. Clinically mild cases referred to case-patients with mild respiratory symptoms without complications, and would not be admitted into hospitals based on routine clinical practice.15–17 Clinical severity measurements included risk for death, risk for death or use of mechanical ventilation, and risk for death or use of mechanical ventilation or ICU admission among hospitalized A(H7N9) case-patients. Clinical severity measurements were estimated using data from hospitalized A(H7N9) patients with definite health outcomes for the first four epidemic waves, while estimated under the competing risk framework considering right-censoring of outcomes for the 5th epidemic wave.
Logistic regression models were used to assess potential factors associated with risk for death among hospitalized A(H7N9) case-patients, excluding patients in the 5th epidemic wave that had unresolved outcomes as of 23 February 2017. We considered risk factors of epidemic wave, age, sex, underlying chronic diseases, place of residence and time delay from illness onset to initiation of antiviral treatment, and we also stratified analyses by wave. As highly pathogenic avian influenza6 A(H7N9) virus formed a monophyletic subclade within lineage C and A(H7N9) lineage B viruses were only detected in Guangdong province,18,19 we added a variable of province to examine whether the hospital-fatality risk differed for cases from Guangdong province and other provinces in the 5th epidemic wave. Adjusted odds ratios (aOR), together with 95% confidence intervals (CI), were used to assess the effects of the risk factors for mortality among hospitalized cases. To examine the linear trend of fatality risk over waves, we conducted a trend test by setting the wave factor as a continuous variable in the logistic regression model.
We used survival analysis to estimate epidemiologic parameters of time-to-event distributions for A(H7N9) cases.20 The time-to-event distributions we assessed were presumed infection to illness onset (incubation period), illness onset to hospital admission, illness onset to initiation of antiviral treatment, hospital admission to death and hospital admission to discharge. Statistical analyses were described in detail in Appendix page 2–3.
Statistical analyses were conducted using R version 3.3.0 (R Foundation for Statistical Computing, Vienna, Austria), and geographic distributions of A(H7N9) human cases were plotted using QGIS (QGIS Development Team, Open Source Geospatial Foundation Project).
Ethical approval
The collection of epidemiological and clinical data for laboratory-confirmed A(H7N9) human cases is a part of a continuing public health investigation of an emerging outbreak, ruled by The National Health and Family Planning Commission. Therefore, our study was exempt from institutional review board assessment.
Role of the funding source
The sponsor of the study had no role in the study design, data collection, data analysis, data interpretation, writing of the report, or the decision to publish. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
RESULTS
The first epidemic wave of human infections with A(H7N9) virus occurred in the spring of 2013 in mainland China, followed by four successive winter-spring epidemic waves from 2013–14, 2014–15, 2015–16 and 2016–17 with laboratory-confirmed cases of 134, 306, 219, 114 and 447, respectively (Table 1, Figure 1A). The number of A(H7N9) cases identified in the 5th epidemic wave was the highest among all epidemic waves and the epidemic wave is still on-going (Figure 1A). The heatmap of wave-specific weekly proportion of laboratory-confirmed A(H7N9) case-patients shows that the 5th epidemic wave started earlier than previous epidemic waves (Figure 1B).
Table 1.
Characteristics of laboratory-confirmed cases of A(H7N9) virus infection by epidemic waves, Mainland China, 2013–2017.
| Characteristic | No. (%) cases | Chi-square statistics | P value | |||||
|---|---|---|---|---|---|---|---|---|
| Wave 1, Jan 2013–Sep 2013, n=134 | Wave 2, Oct 2013–Sep 2014, n=306 | Wave 3, Oct 2014–Sep 2015, n=219 | Wave 4, Oct 2015–Sep 2016, n=114 | Wave 5, Oct 2016–Feb 2017, n=447 | All waves, Jan 2013–Feb 2017, n=1220 | |||
| Median age (range) | 61 (2–91) | 57 (2–88) | 56 (1–89) | 58 (13–92) | 58 (4–93) | 58 (1–93) | ||
| Age group, y | 36·05 | <0·0001 | ||||||
| 0-15 | 7 (5) | 19 (6) | 17 (8) | 2 (2) | 3 (1) | 48 (4) | ||
| 16-59 | 55 (41) | 151 (49) | 116 (53) | 61 (54) | 254 (57) | 637 (52) | ||
| ≥60 | 72 (54) | 136 (44) | 86 (39) | 51 (45) | 190 (43) | 535 (44) | ||
| Male | 94 (70) | 212 (69) | 156 (71) | 77 (68) | 317 (71) | 856 (70) | 0·73 | 0·9478 |
| Presence of at least one underlying medical conditions | 8·57 | 0·0728 | ||||||
| Yes | 59 (44) | 109 (36) | 82 (37) | 55 (48) | 153 (34) | 458 (38) | ||
| No | 59 (44) | 171 (56) | 117 (53) | 54 (47) | 236 (53) | 637 (52) | ||
| Unknown | 16 (12) | 26 (8) | 20 (9) | 5 (4) | 58 (13) | 125 (10) | ||
| Final admitted hospital level | 64·32 | <0·0001 | ||||||
| Provincial level | 78 (58) | 55 (18) | 48 (22) | 14 (12) | 72 (16) | 267 (22) | ||
| Prefecture level | 35 (26) | 136 (44) | 101 (46) | 41 (36) | 145 (32) | 458 (38) | ||
| County/township level | 16 (12) | 37 (12) | 21 (10) | 11 (10) | 38 (9) | 123 (10) | ||
| Unknown | 5 (4) | 78 (25) | 49 (22) | 48 (42) | 192 (43) | 372 (30) | ||
| Residence | 27·90 | 0·0005 | ||||||
| Urban | 81 (60) | 135 (44) | 97 (44) | 41 (36) | 169 (38) | 523 (43) | ||
| Semi-urban | 30 (22) | 97 (32) | 73 (33) | 42 (37) | 140 (31) | 382 (31) | ||
| Rural | 22 (16) | 72 (24) | 49 (22) | 30 (26) | 134 (30) | 307 (25) | ||
| Unknown | 1 (1) | 2 (1) | 0 (0) | 1 (1) | 4 (1) | 8 (1) | ||
| Poultry exposure | ||||||||
| Any exposure to poultry | 1·85 | 0·7633 | ||||||
| Yes | 107 (80) | 209 (68) | 151 (69) | 83 (73) | 337 (75) | 887 (73) | ||
| No | 25 (19) | 46 (15) | 42 (19) | 23 (20) | 74 (17) | 210 (17) | ||
| Unknown | 2 (1) | 51 (17) | 26 (12) | 8 (7) | 36 (8) | 123 (10) | ||
| Visited live poultry market | 7·21 | 0·1254 | ||||||
| Yes | 75 (56) | 165 (54) | 127 (58) | 59 (52) | 269 (60) | 695 (57) | ||
| No | 58 (43) | 100 (33) | 65 (30) | 48 (42) | 142 (32) | 413 (34) | ||
| Unknown | 1 (1) | 41 (13) | 27 (12) | 7 (6) | 36 (8) | 112 (9) | ||
| Exposure to backyard poultry | 41·46 | <0·0001 | ||||||
| Yes | 54 (40) | 51 (17) | 28 (13) | 30 (26) | 107 (24) | 270 (22) | ||
| No | 63 (47) | 175 (57) | 170 (78) | 74 (65) | 311 (70) | 793 (65) | ||
| Unknown | 17 (13) | 80 (26) | 21 (10) | 10 (9) | 29 (6) | 157 (13) | ||
| Exposure to sick or dead poultry | 15·91 | 0·0031 | ||||||
| Yes | 5 (4) | 3 (1) | 11 (5) | 10 (9) | 28 (6) | 57 (5) | ||
| No | 124 (93) | 276 (90) | 181 (83) | 99 (87) | 392 (88) | 1072 (88) | ||
| Unknown | 5 (4) | 27 (9) | 27 (12) | 5 (4) | 27 (6) | 91 (7) | ||
| Occupational exposure to poultry | 8·43 | 0·0770 | ||||||
| Yes | 8 (6) | 22 (7) | 24 (11) | 12 (11) | 25 (6) | 91 (7) | ||
| No | 126 (94) | 279 (91) | 189 (86) | 102 (89) | 417 (93) | 1113 (91) | ||
| Unknown | 0 (0) | 5 (2) | 6 (3) | 0 (0) | 5 (1) | 16 (1) | ||
| Poultry exposure by residence | ||||||||
| Any exposure to poultry | ||||||||
| Yes_Urban | 61 (75) | 82 (61) | 65 (67) | 29 (71) | 126 (75) | 363 (68) | 0·27 | 0·9916 |
| Yes_Semi-urban | 25 (83) | 69 (71) | 49 (67) | 28 (67) | 106 (76) | 277 (71) | 3·15 | 0·5323 |
| Yes_Rural | 20 (91) | 56 (78) | 37 (76) | 25 (83) | 103 (77) | 241 (77) | 3·90 | 0·4192 |
| Visited live poultry market | ||||||||
| Yes_Urban | 50 (62) | 74 (55) | 62 (64) | 26 (63) | 117 (69) | 329 (62) | 5·57 | 0·2333 |
| Yes_Semi-urban | 16 (53) | 51 (53) | 41 (56) | 19 (45) | 86 (61) | 213 (55) | 5·98 | 0·4578 |
| Yes_Rural | 8 (36) | 39 (54) | 24 (49) | 14 (47) | 64 (48) | 149 (47) | 3·63 | 0·0001 |
| Exposure to backyard poultry | ||||||||
| Yes_Urban | 21 (26) | 11 (8) | 5 (5) | 4 (10) | 21 (12) | 62 (12) | 22·84 | <0·0001 |
| Yes_Semi-urban | 15 (50) | 18 (19) | 9 (12) | 10 (24) | 26 (19) | 78 (20) | 19·34 | 0·0007 |
| Yes_Rural | 17 (77) | 22 (31) | 14 (29) | 15 (50) | 60 (45) | 128 (41) | 16·73 | 0·0022 |
| Exposure to sick or dead poultry | ||||||||
| Yes_Urban | 0 (0) | 1 (1) | 2 (2) | 2 (5) | 2 (1) | 7 (1) | 5·36 | 0·2521 |
| Yes_Semi-urban | 2 (7) | 2 (2) | 4 (5) | 1 (2) | 5 (4) | 14 (4) | 2·57 | 0·6323 |
| Yes_Rural | 3 (14) | 0 (0) | 5 (10) | 7 (23) | 21 (16) | 36 (11) | 16·16 | 0·0028 |
| Occupational exposure to poultry | ||||||||
| Yes_Urban | 2 (2) | 6 (4) | 7 (7) | 2 (5) | 5 (3) | 22 (4) | 3·75 | 0·4405 |
| Yes_Semi-urban | 5 (17) | 10 (10) | 7 (10) | 5 (12) | 13 (9) | 40 (10) | 1·49 | 0·0144 |
| Yes_Rural | 1 (5) | 5 (7) | 10 (20) | 5 (17) | 7 (5) | 28 (9) | 12·43 | <0·0001 |
| Treatment | ||||||||
| Antiviral | 4·63 | 0·3269 | ||||||
| Yes | 79 (59) | 247 (81) | 178 (81) | 94 (82) | 310 (69) | 908 (74) | ||
| No | 5 (4) | 15 (5) | 13 (6) | 7 (6) | 10 (2) | 50 (4) | ||
| Unknown | 50 (37) | 44 (14) | 28 (13) | 13 (11) | 127 (28) | 262 (21) | ||
| Antibiotic | 9·10 | 0·0588 | ||||||
| Yes | 103 (77) | 269 (88) | 188 (86) | 102 (89) | 318 (71) | 980 (80) | ||
| No | 4 (3) | 16 (5) | 8 (4) | 3 (3) | 4 (1) | 35 (3) | ||
| Unknown | 27 (20) | 21 (7) | 23 (11) | 9 (8) | 125 (28) | 205 (17) | ||
| Corticosteroid | 19·95 | 0·0005 | ||||||
| Yes | 38 (28) | 138 (45) | 118 (54) | 70 (61) | 203 (45) | 567 (46) | ||
| No | 38 (28) | 87 (28) | 56 (26) | 17 (15) | 94 (21) | 292 (24) | ||
| Unknown | 58 (43) | 81 (26) | 45 (21) | 27 (24) | 150 (34) | 361 (30) | ||
| Oxygen | 26·11 | <0·0001 | ||||||
| Yes | 120 (90) | 251 (82) | 158 (72) | 101 (89) | 291 (65) | 921 (75) | ||
| No | 11 (8) | 33 (11) | 30 (14) | 2 (2) | 15 (3) | 91 (7) | ||
| Unknown | 3 (2) | 22 (7) | 31 (14) | 11 (10) | 141 (32) | 208 (17) | ||
| Mechanical ventilation | 3·34 | 0·5028 | ||||||
| Yes | 73 (54) | 133 (43) | 111 (51) | 62 (54) | 185 (41) | 564 (46) | ||
| No | 47 (35) | 113 (37) | 75 (34) | 40 (35) | 118 (26) | 393 (32) | ||
| Unknown | 14 (10) | 60 (20) | 33 (15) | 12 (11) | 144 (32) | 263 (22) | ||
| ECMO | 12·38 | 0·0147 | ||||||
| Yes | 14 (10) | 8 (3) | 11 (5) | 4 (4) | 25 (6) | 62 (5) | ||
| No | 103 (77) | 237 (77) | 168 (77) | 95 (83) | 278 (62) | 881 (72) | ||
| Unknown | 17 (13) | 61 (20) | 40 (18) | 15 (13) | 144 (32) | 277 (23) | ||
| ICU | 8·97 | 0·0618 | ||||||
| Yes | 89 (66) | 153 (50) | 129 (59) | 71 (62) | 234 (52) | 676 (55) | ||
| No | 34 (25) | 84 (27) | 53 (24) | 29 (25) | 73 (16) | 273 (22) | ||
| Unknown | 11 (8) | 69 (23) | 37 (17) | 14 (12) | 140 (31) | 271 (22) | ||
| Outcome | 8·40 | 0·0780 | ||||||
| Death | 45 (34) | 131 (43) | 102 (47) | 47 (41) | 169 (38) | 494 (40) | ||
| Recover | 89 (66) | 175 (57) | 117 (53) | 64 (56) | 188 (42) | 633 (52) | ||
| Unknown/Unresolveda | 0 (0) | 0 (0) | 0 (0) | 3 (3) | 90 (20) | 93 (8) | ||
The health outcomes for cases in wave 4 were unknown because of loss to follow up, while the health outcomes for cases in wave 5 were unresolved as they were still in hospital when we retrieved the data.
Abbreviations: ECMO, extra-corporeal membrane oxygenation; ICU, intensive care unit.
Figure 1. Temporal pattern of laboratory-confirmed cases of A(H7N9) virus infection, Mainland China, 2013–2017.
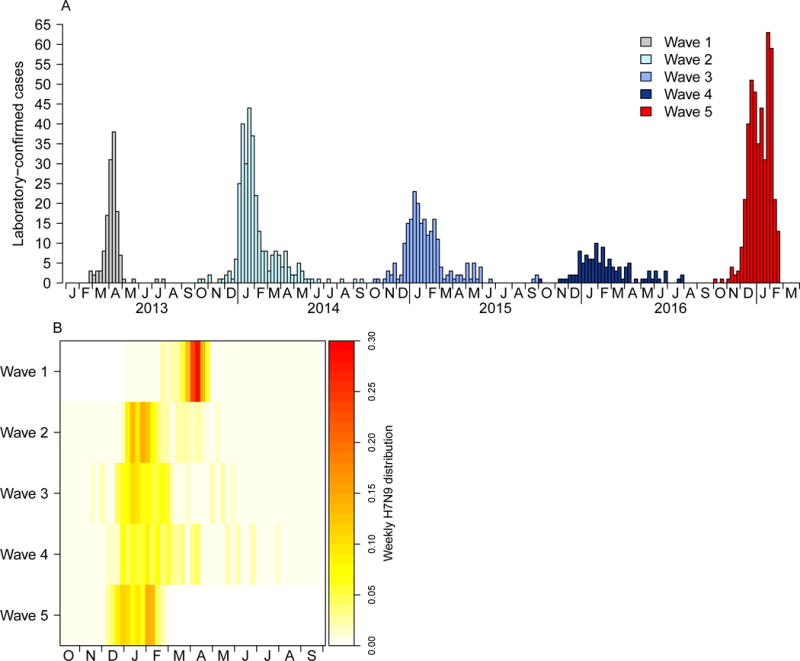
A) Epidemic curve of cases by week; B) Heatmap of weekly proportion of laboratory-confirmed cases by waves.
A total of 20/31 provinces have reported the identification of human cases of A(H7N9) virus infection, and cases were concentrated in the Yangtze River Delta in eastern China and Guangdong province in southern China (Figure 2 A–B; Appendix, page 4). Although Sichuan province was the only new province reporting laboratory-confirmed human case of A(H7N9) virus infection in the 5th epidemic wave, there were 144 newly affected counties (Figure 2C; Appendix, page 5–6). Zhejiang, Guangdong and Jiangsu were the three provinces with the highest cumulative case numbers of 294, 247 and 232, respectively.
Figure 2. Geographic distribution of laboratory-confirmed cases of A(H7N9) virus infection in Mainland China, 2013–2017.
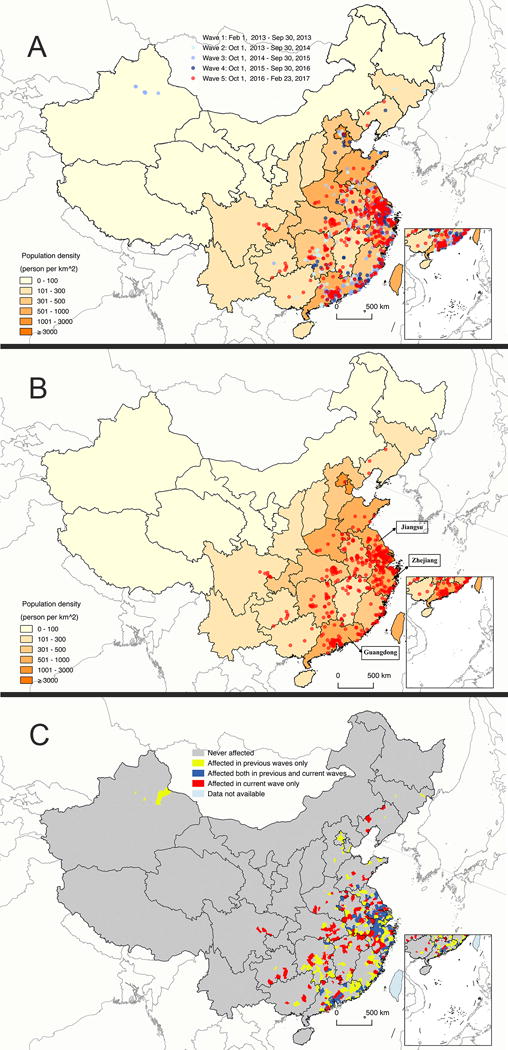
A) All waves; B) Wave 5; C) Counties affected by A(H7N9) across 5 epidemic waves.
The median age of laboratory-confirmed human infections with A(H7N9) virus in the 5th epidemic wave was 58 years, which was comparable to previous epidemic waves (Table 1). However, the proportion of cases under 16 years was reduced to 1% in the 5th epidemic wave with an increase in the proportion of cases aged 16–59 years (Table 1, Figure 3A). The difference in age distribution across epidemic waves was statistically significant (Table 1), although the 0–15 years age group was sparse and the 16–59 years age group was the only group with a consistent increasing proportion over the waves. Men consistently accounted for around 70% of all cases during the five epidemic waves (Table 1), while the age and sex distribution varied slightly across epidemic waves (Figure 3 B–G). Over half of the cases were finally admitted into a provincial hospital in the 1st epidemic wave, while cases were more likely to be admitted into prefecture level hospitals in subsequent epidemic waves (Table 1). Antiviral, antibiotic and corticosteroid treatments, oxygen treatment and mechanical ventilation were commonly administered for A(H7N9) case-patients, and less than 10% A(H7N9) case-patients received ECMO (Table 1). 34%–47% A(H7N9) case-patients died in the five epidemic waves (Table 1). Residence of A(H7N9) case-patients shifted gradually from urban to semi-urban and rural areas from the 1st to the 5th epidemic wave with a statistically significant difference in case residence across epidemic waves, although the situation varied by province (Table 1, Appendix page 7).
Figure 3. Age and sex distributions of laboratory-confirmed cases of A(H7N9) virus infection by five epidemic waves, Mainland China, 2013–2017.
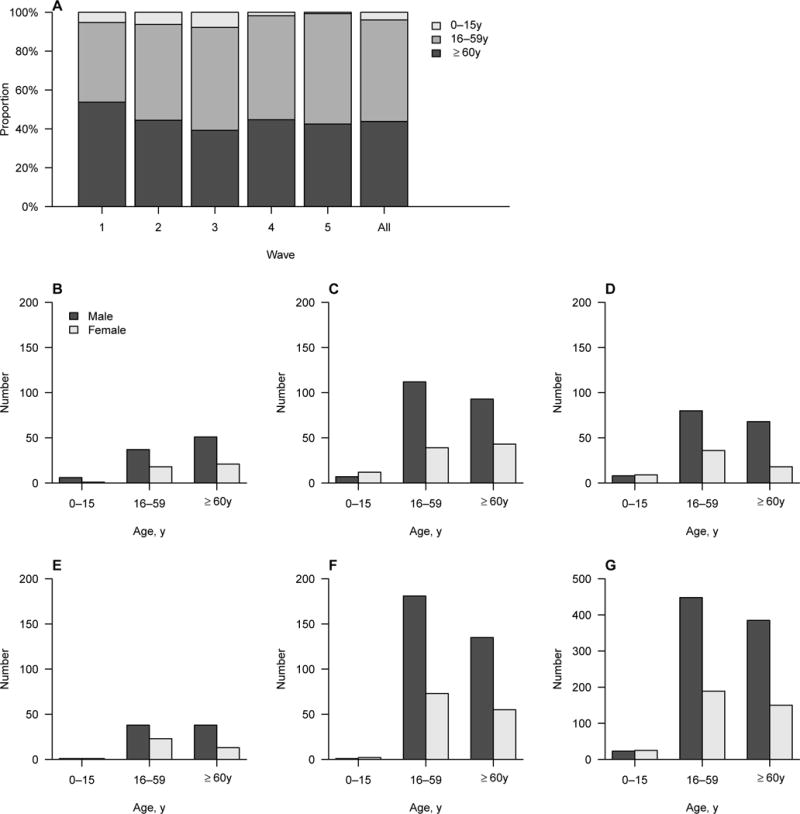
A) Age distribution; B–G) Age and sex distribution in wave 1–5 and all waves.
Around 70% of human cases of laboratory-confirmed A(H7N9) virus infection reported exposure to poultry within the 10 days before the onset of symptoms across five epidemic waves (Table 1). Visiting a live poultry market and exposure to backyard poultry were the two major sources of poultry exposure (Table 1). Although the chance of poultry exposure from visiting a live poultry market was lower for rural cases than urban and semi-urban cases, around 40% of rural A(H7N9) case-patients reported poultry exposure from visiting a live poultry market (Table 1, Appendix page 7). For cases with occupational poultry exposures, semi-urban cases reported a higher proportion of exposure than urban and rural cases in the 1st, 2nd, 5th and total epidemic waves (Table 1, Appendix page 7).
Clinical severity for hospitalized A(H7N9) case-patients increased from the 1st to subsequent epidemic waves (Figure 4A). A linear increase in fatality risk was observed from the younger age groups to older age groups (Figure 4B). Case-patients aged over 60 years consistently had higher risks for death, death/mechanical ventilation, death/mechanical ventilation/ICU admission than case-patients aged below 60 years (Figure 4 C–E). After adjusting for age, sex, underlying medical conditions, case residence and delay from illness onset to antiviral treatment, the aOR for fatality risk increased by epidemic waves except for the 4th epidemic wave (Table 2), and trend test for wave was statistically significant (P=0·0342). Case-patients with older age had higher risk for death, and the aORs increased from 0·70 (95% CI, 0·03–6·38) for 0–15 years, 1 for 16–59 years (reference group), 1·84 (95% CI, 1·28–2·65) for 60–74 years to 2·28 (95% CI, 1·43–3·68) for ≥75 years, and trend test for age was statistically significant (P<0·0001). Case-patients with underlying medical conditions or that received antiviral treatment >48 hours after symptom onset consistently had higher risks for death, although the aORs were not statistically significant (Table 2). In the 5th epidemic wave, case-patients from Guangdong province had a slightly lower risk for death, but this was not statistically significant (Table 2). The median days from illness onset to initiation of antiviral treatment were 5·5, 6·2, 5·4, 4·4 and 4·8 days from wave 1 to wave 5, respectively (Appendix, page 8).
Figure 4. Clinical severity profile for hospitalized laboratory-confirmed cases of A(H7N9) virus infection.
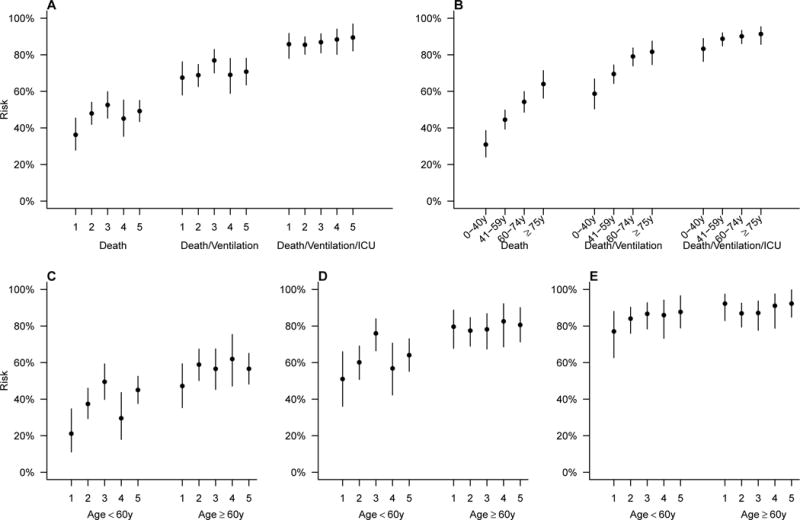
A) Risk for deaths, death/mechanical ventilation, death/mechanical ventilation/ICU admissions by waves; B) Risk for deaths, death/mechanical ventilation, death/mechanical ventilation/ICU admissions by age groups; C) Risk for deaths by waves and age groups; D) Risk for deaths/mechanical ventilation by waves and age groups; E) Risk for deaths/mechanical ventilation/ICU admissions by waves and age groups. (ICU, intensive care unit)
Table 2.
Comparison of risk for death among hospitalized patients with laboratory-confirmed A(H7N9) virus infection by epidemic waves, mainland China, 2013–2017.
| Characteristic | All waves | Wave 1 | Wave 2 | Wave 3 | Wave 4 | Wave 5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| aOR (95% CI) | P value | aOR (95% CI) | P value | aOR (95% CI) | P value | aOR (95% CI) | P value | aOR (95% CI) | P value | aOR (95% CI) | P value | |
| Age group, y | ||||||||||||
| 0-59 | 1·00 | 1·00 | 1·00 | 1·00 | 1·00 | 1·00 | ||||||
| ≥60 | 1·96 (1·42,2·72) | <0·0001 | 2·29 (0·71,8·05) | 0·1735 | 2·55 (1·31,5·11) | 0·0066 | 1·29 (0·64,2·62) | 0·4673 | 5·79 (1·97,19·0) | 0·0021 | 1·61 (0·91,2·88) | 0·0995 |
| Sex | ||||||||||||
| Female | 1·00 | 1·00 | 1·00 | 1·00 | 1·00 | 1·00 | ||||||
| Male | 0·88 (0·63,1·24) | 0·4859 | 0·43 (0·12,1·42) | 0·1722 | 0·80 (0·38,1·65) | 0·5608 | 0·76 (0·35,1·63) | 0·4963 | 1·53 (0·48,4·94) | 0·4634 | 0·88 (0·48,1·58) | 0·6772 |
| Underlying medical conditions | ||||||||||||
| No | 1·00 | 1·00 | 1·00 | 1·00 | 1·00 | 1·00 | ||||||
| Yes | 1·32 (0·96,1·83) | 0·0812 | 0·99 (0·30,3·29) | 0·9867 | 0·88 (0·45,1·71) | 0·7245 | 1·33 (0·67,2·66) | 0·4116 | 1·31 (0·44,3·83) | 0·6095 | 1·72 (0·98,3·07) | 0·0595 |
| Residence | ||||||||||||
| Urban | 1·00 | 1·00 | 1·00 | 1·00 | 1·00 | 1·00 | ||||||
| Semi-urban | 0·95 (0·65,1·38) | 0·7942 | 1·17 (0·23,5·53) | 0·8416 | 1·19 (0·53,2·73) | 0·6609 | 1·33 (0·62,2·85) | 0·4562 | 0·15 (0·04,0·51) | 0·0034 | 1·22 (0·63,2·37) | 0·5409 |
| Rural | 1·02 (0·69,1·52) | 0·9023 | 0·30 (0·01,2·28) | 0·3118 | 1·43 (0·64,3·26) | 0·3816 | 1·06 (0·45,2·49) | 0·8766 | 0·19 (0·04,0·71) | 0·0173 | 1·56 (0·79,3·12) | 0·1998 |
| Onset to antiviral treatment, d | ||||||||||||
| 0-2 | 1·00 | 1·00 | 1·00 | 1·00 | 1·00 | 1·00 | ||||||
| ≥3 | 1·52 (0·91,2·57) | 0·1074 | 1·38 (0·19,12·4) | 0·7476 | 1·68 (0·37,7·72) | 0·4855 | 1·66 (0·53,5·46) | 0·3807 | 1·79 (0·41,8·49) | 0·4401 | 1·39 (0·62,3·15) | 0·4146 |
| Province | ||||||||||||
| Province other than Guangdong | 1·00 | |||||||||||
| Guangdong | 0·86 (0·41,1·81) | 0·7048 | ||||||||||
| Wave | ||||||||||||
| 1 | 0·53 (0·26,1·07) | 0·0822 | ||||||||||
| 2 | 1·25 (0·72,2·16) | 0·4240 | ||||||||||
| 3 | 1·36 (0·78,2·38) | 0·2667 | ||||||||||
| 4 | 1·00 | |||||||||||
| 5 | 1·48 (0·88,2·50) | 0·1380 | ||||||||||
Abbreviation: aOR, adjusted odds ratio.
DISCUSSION
Our study presented the temporal pattern and spatial dissemination of A(H7N9) epidemics in China, and provided a comprehensive description of the epidemiology of laboratory-confirmed cases of A(H7N9) virus infection, and assessed the clinical severity of A(H7N9) case-patients during the 5th epidemic wave. The 5th epidemic wave occurred earlier, spread to more counties in affected provinces and infected more people compared to previous epidemic waves. Although there was only one additional province (Sichuan province) reporting human infections with A(H7N9) virus in the 5th epidemic wave as of February 2017, an analysis at the county level suggested much wider geographic spread with many newly affected counties, and the 5th epidemic wave is on-going. Human infections with A(H7N9) virus shifted gradually from urban to semi-urban and rural areas, although the shift differed by provinces.
The earlier occurrence of the 5th epidemic wave may be associated with changes in environmental factors such as weather conditions, and more research is needed to elucidate the mechanisms for seasonality. The geographic expansion of human infections with A(H7N9) virus suggests that A(H7N9) viruses circulating among poultry have spread more widely in China since the emergence of human cases in 2013. The majority of human infections with A(H7N9) virus to date have been with a low pathogenic avian influenza (LPAI) A virus until recently as some virus strains have evolved to be highly pathogenic in the 5th epidemic wave. Since LPAI A(H7N9) virus does not cause illness in infected poultry, prevention and containment can be very challenging. LPAI A(H7N9) virus may be undetected in poultry farms, and wholesale and retail markets, unless active surveillance is conducted.21 Moreover, as increasing numbers of live poultry markets were closed in counties where laboratory-confirmed A(H7N9) cases were reported, it might have resulted in movement of ready-for-market poultry infected with LPAI A(H7N9) virus to wider geographic areas without or with less strict implementation of market closure, including previously unaffected counties. Hence, we suspect that LPAI A(H7N9) virus could spread silently along the poultry trade and transportation routes, although we do not have data on trans-regional poultry trade in China.
Laboratory-confirmed cases with A(H7N9) virus infection reported similar low frequencies of exposure to sick/dead poultry across the five epidemic waves. However, the exposure pattern may change in the future depending upon the proportion of low and highly pathogenic avian influenza A(H7N9) viruses circulating among poultry in China. On-going assessment of poultry exposures is essential to study the changes in exposure risk, especially to sick/dead poultry. Although the pattern of poultry exposures for reported human infections with A(H7N9) virus has not changed substantially, the prevalence of poultry infected with A(H7N9) viruses is probably higher than before, as more human infections were reported during the 5th epidemic wave.
Although the risk for death showed an increasing trend across waves, no statistically significant risk difference was observed when comparing the 5th epidemic wave to the 4th epidemic wave. Avian influenza A(H7N9) viruses have undergone some genetic changes,6 but our findings suggest that the clinical severity of A(H7N9) case-patients in the 5th epidemic wave remained similar to the previous epidemic wave. However, if we set the 1st epidemic wave as the reference group, we could observe the adjusted ORs from the logistic regression model were statistically significant for the 2nd, 3rd and 5th epidemic waves. The increased clinical severity after the first epidemic wave could result from case ascertainment bias. After announcement of the first human case of A(H7N9) virus infection in the 1st epidemic wave, both the government and the public were extremely concerned about this novel avian influenza A virus. For example, people became more worried than usual if they developed influenza-like illness (ILI), especially in urban areas.22 Laboratory testing for A(H7N9) virus was conducted intensively among suspected cases in the 1st epidemic wave. It is possible that after 2013, an improved public perception of a lower risk of A(H7N9) virus infection may have changed people’s health seeking behavior. We found that the initiation of antiviral treatment within 48 hours after illness onset was associated with a lower risk for death than the delayed treatment beyond 48 hours since symptom onset. Although antiviral treatment started slightly earlier for A(H7N9) case-patients identified in the latest two epidemic waves than the first three epidemic waves, a reduced fatality risk was not observed in the hospitalized patients detected in recent waves.
Although clusters of human cases have been reported, with epidemiological evidence suggesting some instances of limited human-to-human transmission,14 there has been no evidence of sustained human-to-human A(H7N9) virus transmission to date. Poultry-to-human transmission remains the major transmission route, and high proportions of laboratory-confirmed cases of A(H7N9) virus infection reported poultry exposure by visiting live poultry markets. Environmental surveillance conducted in Zhejiang province during 2015–16 revealed that 17·3% of the environmental samples collected from live poultry markets were contaminated by H7 subtype viruses.23 Closure of live poultry markets has been demonstrated to be highly effective in reducing the risk of human infection with A(H7N9) virus by reducing poultry exposures.24,25 Although some cities have temporarily or permanently closed live poultry markets, there are still reported A(H7N9) cases from these cities. Although closure of large live poultry markets may have been implemented in central urban areas, it is possible that smaller live poultry markets in semi-urban and rural areas may still be operating. Epidemiological investigations revealed that more than 40% of semi-urban and rural cases reported that they had visited a live poultry market before illness onset. Closure of live poultry markets in many areas may have been implemented too late in the 5th epidemic wave, and only after many A(H7N9) cases had been identified. Regular closure of live poultry markets before the annual winter A(H7N9) epidemic period could be a more effective prevention measure than closure after A(H7N9) cases have been identified.21 The expansion of areas with permanent closure of live poultry markets might also help to further reduce A(H7N9) cases in the future.
Zhejiang, Jiangsu and Guangdong were the three provinces with the highest cumulative numbers of reported human infections with A(H7N9) virus since early 2013. We observed a weak association between the incidence of A(H7N9) human infections and chicken/duck density,26 which may be confounded by chicken breeds. Yellow chicken is produced and consumed much more commonly then white chicken in the southern part of the country. Further investigation of factors associated with the persistence of A(H7N9) virus among poultry in these three provinces is necessary to inform prevention and control measures to reduce the risk of human cases. Risk mapping of A(H7N9) virus in the Yangtze River Delta and Pearl River Delta may provide evidence for making future preventive strategies.
Sentinel surveillance for ILI contributes to identification of clinically mild A(H7N9) cases. The ILI surveillance network consists of 554 sentinel hospitals across 31 provinces, covering 2·5% of all hospitals in China. For each sentinel hospital, 10–15 respiratory specimens were collected each week by convenience samples of patients with ILI symptoms. Considering the sampling method currently used for sentinel surveillance, a large number of undetected A(H7N9) cases with mild symptoms could be occuring.15 Identification of clinically mild cases through ILI surveillance reveals the “iceberg under the sea”, with implications for the estimation of the infection-fatality risk (which may be much lower than the hospitalization-fatality risk estimated in our study) and the total number of A(H7N9) virus infections that have occurred.15,27 However, the true denominator of human A(H7N9) virus infections to date, including asymptomatic and clinically mild illness in China, to better inform severity assessments, is unknown.
Our study had several limitations. First, our study analyzed only laboratory-confirmed human infections with A(H7N9) virus that were hospitalized and excluded clinically mild cases. Therefore, the full clinical spectrum of human infection with A(H7N9) virus was not assessed. Second, our estimates for clinical severity could be biased if there were undetected hospitalized patients with A(H7N9) virus infection that were not suspected by clinicians and therefore not tested for A(H7N9) virus infection, or due to lack of access to laboratory testing in some areas. Third, it is possible that the cases or their family members may not have accurately recalled and reported exposure to poultry or to live poultry markets, which might affect estimation of the incubation period. Fourth, some cases did not have an exact date of hospital admission reported in the China CDC A(H7N9) case database, which could result in a slightly biased estimation of the clinical severity.
In conclusion, the 5th epidemic wave of human cases of A(H7N9) virus infection occurred earlier, spread to more areas and infected more people compared to previous epidemic waves. Cases shifted from the elderly to middle-aged adults and from urban locations to semi-urban and rural areas. The clinical severity remains unchanged to date. Continuous monitoring and regular assessments of the epidemiology characteristics and clinical severity of human infections with avian influenza A(H7N9) virus are necessary for pandemic risk assessment.
Supplementary Material
Panel.
Evidence before this study
Avian influenza A(H7N9) virus merged in 2013 and poses a pandemic threat to human population. Human cases of avian influenza A(H7N9) virus infection have occurred during annual winter-spring epidemic waves in mainland China since 2013. A sudden increase in human infections with avian influenza A(H7N9) virus was observed in December 2016 in mainland China, prompting concerns of whether the epidemiology had changed to suggest an increasing pandemic threat. On 13 March 2017, we searched the PubMed for the terms “H7N9” and “epidemiology*” in title/abstract, restricting the publication date after 1 October 2016. We identified 12 English language publications, but none described the 5th A(H7N9) epidemic wave after reviewing the title and abstract. We have been continuously tracking the publications of A(H7N9) epidemiology characteristics and clinical assessment since 1 October 2016. We knew of an article published in the Western Pacific Surveillance Response Journal that described the epidemic curve of human infections with A(H7N9) virus up to December 2016 in China. However, human infections had not reached the peak when that paper was published. We also knew of a recent article published in the Morbidity and Mortality Weekly Report reporting human cases infected with A(H7N9) virus until February 2017, but no epidemiological characteristics were reported. No study assessing the clinical severity of A(H7N9) virus infection for the 5th epidemic wave was identified.
Added value of this study
Our study presented the temporal pattern and spatial dissemination of A(H7N9) epidemics across epidemic waves. The 5th epidemic wave started earlier, spread to more counties in affected provinces and infected more people compared to previous waves. Detailed epidemiological data for the laboratory-confirmed human infections with A(H7N9) virus in the 5th epidemic wave were provided and compared with previous epidemic waves. Residence of A(H7N9) case-patients shifted gradually from urban to semi-urban and rural areas from the 1st to the 5th epidemic, although the situation varied by province. The poultry exposure risk has not changed substantially. The clinical severity of human infections with A(H7N9) virus among hospitalized cases remained similar to previous three epidemic waves.
Implications of all the available evidence
This is the first study that provided detailed epidemiological characteristics and assessed the clinical severity for hospitalized cases of human infection with A(H7N9) virus in the 5th epidemic wave. Regular closure of live poultry markets before annual winter A(H7N9) epidemics and expansion of areas with permanent closure of live poultry markets are recommended. Continued vigilance and sustained intensive control efforts are needed to minimize the risk of human infection with A(H7N9) virus.
Acknowledgments
We thank staff members at county-, prefecture-, and provincial-level Centers for Disease Control and Prevention in the provinces where human cases of avian influenza A(H7N9) virus infection occurred for providing assistance with field investigation, administration and data collection.
Funding
This study was funded by grants from the National Science Fund for Distinguished Young Scholars (grant no. 81525023), the US National Institutes of Health (grant no. U19 AI51915; R01AI101028-02A1), China CDC’s Key Laboratory of Surveillance and Early-warning on Infectious Disease, the Harvard Center for Communicable Disease Dynamics from the National Institute of General Medical Sciences (grant no. U54 GM088558), a grant from the Health and Medical Research Fund of the Health, Welfare and Food Bureau of the Hong Kong SAR Government (grant no. 14131432), and the Research Grants Council of the Hong Kong Special Administrative Region, China (Project No. T11-705/14N), and a commissioned grant from the Health and Medical Research Fund of the Health, Welfare and Food Bureau of the Hong Kong SAR Government, the National Natural Science Foundation of China (grant no. 81402731; 81602936), and Natural Science Foundation of Shanghai (grant no. 14ZR1444500). The funding bodies had no role in study design, data collection and analysis, preparation of the manuscript, or the decision to publish.
Footnotes
Conflicts of interest
BJC has received research funding from Sanofi Pasteur. The authors report no other potential conflicts of interest.
Disclaimer: The views expressed are those of the authors and do not necessarily represent the official policy of the Chinese Center for Disease Control and Prevention or the U.S. Centers for Disease Control and Prevention.
Contributors
HY conceived, designed, and supervised the study. HJ, LF, LW, XH, KX, EC, XW, JH, MK, RZ, JZ, JW, SH, HZ, XL, WF, JO and SW collected data. YQ, YS, JZ and XW cleaned data. XW, PW and VF analyzed the data. JZ, SL and ZZ mapped the case geographic distribution. XW, PW, BC, TU and HY wrote the drafts of the manuscript. HY, BC, TU, JA and MG interpreted the findings. HZ, YG, GG, PWH and GL commented on and revised drafts of the manuscript. All authors read and approved the final manuscript.
Contributor Information
Xiling Wang, School of Public Health, Fudan University, Key Laboratory of Public Health Safety, Ministry of Education, Shanghai, China.
Hui Jiang, Key Laboratory of Surveillance and Early-warning on Infectious Disease, Division of Infectious Disease, Chinese Center for Disease Control and Prevention, Beijing, China.
Peng Wu, WHO Collaborating Centre for Infectious Disease Epidemiology and Control, School of Public Health, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong Special Administrative Region, China.
Timothy M. Uyeki, Influenza Division, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, GA, USA.
Luzhao Feng, Key Laboratory of Surveillance and Early-warning on Infectious Disease, Division of Infectious Disease, Chinese Center for Disease Control and Prevention, Beijing, China.
Shengjie Lai, Key Laboratory of Surveillance and Early-warning on Infectious Disease, Division of Infectious Disease, Chinese Center for Disease Control and Prevention, Beijing, China.
Lili Wang, Institute Pasteur of Shanghai, Chinese Academy of Sciences, Shanghai, China.
Xiang Huo, Jiangsu Provincial Center for Disease Control and Prevention, Suzhou, China.
Ke Xu, Jiangsu Provincial Center for Disease Control and Prevention, Suzhou, China.
Enfu Chen, Zhejiang Provincial Center for Disease Control and Prevention, Hangzhou, China.
Xiaoxiao Wang, Zhejiang Provincial Center for Disease Control and Prevention, Hangzhou, China.
Jianfeng He, Guangdong Provincial Center for Disease Control and Prevention, Guangzhou, China.
Min Kang, Guangdong Provincial Center for Disease Control and Prevention, Guangzhou, China.
Renli Zhang, Shenzhen Center for Disease Control and Prevention, Shenzhen, China.
Jin Zhang, Anhui Provincial Center for Disease Control and Prevention, Hefei, China.
Jiabing Wu, Anhui Provincial Center for Disease Control and Prevention, Hefei, China.
Shixiong Hu, Hunan Provincial Center for Disease Control and Prevention, Changsha, China.
Hengjiao Zhang, Hunan Provincial Center for Disease Control and Prevention, Changsha, China.
Xiaoqing Liu, Jiangxi Provincial Center for Disease Control and Prevention, Nanchang, China.
Weijie Fu, Jiangxi Provincial Center for Disease Control and Prevention, Nanchang, China.
Jianming Ou, Fujian Provincial Center for Disease Control and Prevention, Fuzhou, China.
Shenggen Wu, Fujian Provincial Center for Disease Control and Prevention, Fuzhou, China.
Ying Qin, Key Laboratory of Surveillance and Early-warning on Infectious Disease, Division of Infectious Disease, Chinese Center for Disease Control and Prevention, Beijing, China.
Zhijie Zhang, School of Public Health, Fudan University, Key Laboratory of Public Health Safety, Ministry of Education, Shanghai, China.
Yujing Shi, Key Laboratory of Surveillance and Early-warning on Infectious Disease, Division of Infectious Disease, Chinese Center for Disease Control and Prevention, Beijing, China.
Juanjuan Zhang, School of Public Health, Fudan University, Key Laboratory of Public Health Safety, Ministry of Education, Shanghai, China.
Jean Artois, Spatial Epidemiology Lab. (SpELL), ‘Université Libre de Bruxelles’, Brussels, Belgium.
Vicky J. Fang, WHO Collaborating Centre for Infectious Disease Epidemiology and Control, School of Public Health, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong Special Administrative Region, China.
Huachen Zhu, WHO Collaborating Centre for Infectious Disease Epidemiology and Control, School of Public Health, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong Special Administrative Region, China; Centre of Influenza Research and State Key Laboratory of Emerging Infectious Diseases, School of Public Health, The University of Hong Kong, Hong Kong Special Administrative Region, China; State Key Laboratory of Emerging Infectious Diseases (The University of Hong Kong-Shenzhen Branch), Shenzhen Third People’s Hospital, Shenzhen, China; Shantou University-The University of Hong Kong Joint Institute of Virology, Shantou University, Shantou, China.
Yi Guan, WHO Collaborating Centre for Infectious Disease Epidemiology and Control, School of Public Health, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong Special Administrative Region, China; Centre of Influenza Research and State Key Laboratory of Emerging Infectious Diseases, School of Public Health, The University of Hong Kong, Hong Kong Special Administrative Region, China; State Key Laboratory of Emerging Infectious Diseases (The University of Hong Kong-Shenzhen Branch), Shenzhen Third People’s Hospital, Shenzhen, China; Shantou University-The University of Hong Kong Joint Institute of Virology, Shantou University, Shantou, China.
Marius Gilbert, Spatial Epidemiology Lab. (SpELL), ‘Université Libre de Bruxelles’, Brussels, Belgium; Fonds National de la Recherche Scientifique, Brussels, Belgium.
Peter W. Horby, Centre for Tropical Medicine and Global Health, Nuffield Department of Medicine, University of Oxford, Oxford, UK.
Gabriel M. Leung, WHO Collaborating Centre for Infectious Disease Epidemiology and Control, School of Public Health, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong Special Administrative Region, China.
George F. Gao, Centre of Influenza Research and State Key Laboratory of Emerging Infectious Diseases, School of Public Health, The University of Hong Kong, Hong Kong Special Administrative Region, China; Chinese Center for Disease Control and Prevention, Beijing, China.
Benjamin J. Cowling, WHO Collaborating Centre for Infectious Disease Epidemiology and Control, School of Public Health, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong Special Administrative Region, China.
Hongjie Yu, School of Public Health, Fudan University, Key Laboratory of Public Health Safety, Ministry of Education, Shanghai, China.
References
- 1.Gao R, Cao B, Hu Y, et al. Human infection with a novel avian-origin influenza A (H7N9) virus. N Engl J Med. 2013;368(20):1888–97. doi: 10.1056/NEJMoa1304459. [DOI] [PubMed] [Google Scholar]
- 2.Zhou L, Ren R, Yang L, et al. Sudden increase in human infection with avian influenza A(H7N9) virus in China, September–December 2016. Western Pac Surveill Response J. 2017;8(1) doi: 10.5365/WPSAR.2017.8.1.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iuliano AD, Jang Y, Jones J, et al. Increase in Human Infections with Avian Influenza A(H7N9) Virus During the Fifth Epidemic — China, October 2016–February 2017. MMWR Morb Mortal Wkly Rep. 2017;66 doi: 10.15585/mmwr.mm6609e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Riel D, Leijten LM, de Graaf M, et al. Novel avian-origin influenza A (H7N9) virus attaches to epithelium in both upper and lower respiratory tract of humans. Am J Pathol. 2013;183(4):1137–43. doi: 10.1016/j.ajpath.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan MC, Chan RW, Chan LL, et al. Tropism and innate host responses of a novel avian influenza A H7N9 virus: an analysis of ex-vivo and in-vitro cultures of the human respiratory tract. Lancet Respir Med. 2013;1(7):534–42. doi: 10.1016/S2213-2600(13)70138-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chinese Center for Disease Control and Prevention. Mutations on H7N9 virus have been identified in H7N9 patients in China. 2017 http://www.chinacdc.cn/gwswxx/yjzx/201702/t20170219_138185.html (accessed 19 February 2017)
- 7.Yu H, Cowling BJ, Feng L, et al. Human infection with avian influenza A H7N9 virus: an assessment of clinical severity. Lancet. 2013;382(9887):138–45. doi: 10.1016/S0140-6736(13)61207-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng L, Wu JT, Liu X, et al. Clinical severity of human infections with avian influenza A(H7N9) virus, China, 2013/14. Euro Surveill. 2014;19(49) doi: 10.2807/1560-7917.es2014.19.49.20984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu P, Peng Z, Fang VJ, et al. Human Infection with Influenza A(H7N9) Virus during 3 Major Epidemic Waves, China, 2013-2015. Emerg Infect Dis. 2016;22(6):964–72. doi: 10.3201/eid2206.151752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Q, Zhou L, Zhou M, et al. Epidemiology of human infections with avian influenza A(H7N9) virus in China. N Engl J Med. 2014;370(6):520–32. doi: 10.1056/NEJMoa1304617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiang N, Iuliano AD, Zhang Y, et al. Comparison of the first three waves of avian influenza A(H7N9) virus circulation in the mainland of the People’s Republic of China. BMC Infect Dis. 2016;16(1):734. doi: 10.1186/s12879-016-2049-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiang N, Li X, Ren R, et al. Assessing Change in Avian Influenza A(H7N9) Virus Infections During the Fourth Epidemic - China, September 2015-August 2016. MMWR Morb Mortal Wkly Rep. 2016;65(49):1390–4. doi: 10.15585/mmwr.mm6549a2. [DOI] [PubMed] [Google Scholar]
- 13.Cowling BJ, Jin L, Lau EH, et al. Comparative epidemiology of human infections with avian influenza A H7N9 and H5N1 viruses in China: a population-based study of laboratory-confirmed cases. Lancet. 2013;382(9887):129–37. doi: 10.1016/S0140-6736(13)61171-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qin Y, Horby PW, Tsang TK, et al. Differences in the Epidemiology of Human Cases of Avian Influenza A(H7N9) and A(H5N1) Viruses Infection. Clin Infect Dis. 2015;61(4):563–71. doi: 10.1093/cid/civ345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ip DK, Liao Q, Wu P, et al. Detection of mild to moderate influenza A/H7N9 infection by China’s national sentinel surveillance system for influenza-like illness: case series. BMJ. 2013;346:f3693. doi: 10.1136/bmj.f3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiang N, Havers F, Chen T, et al. Use of national pneumonia surveillance to describe influenza A(H7N9) virus epidemiology, China, 2004-2013. Emerg Infect Dis. 2013;19(11):1784–90. doi: 10.3201/eid1911.130865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu C, Havers F, Wang L, et al. Monitoring avian influenza A(H7N9) virus through national influenza-like illness surveillance, China. Emerg Infect Dis. 2013;19(8):1289–92. doi: 10.3201/eid1908.130662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lam TT, Zhou B, Wang J, et al. Dissemination, divergence and establishment of H7N9 influenza viruses in China. Nature. 2015;522(7554):102–5. doi: 10.1038/nature14348. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization. Antigenic and genetic characteristics of zoonotic influenza viruses and candidate vaccine viruses developed for potential use in human vaccines. 2017 http://www.who.int/entity/influenza/vaccines/virus/201703_zoonotic_vaccinevirusupdate.pdf?ua=1 (accessed 18 March 2017)
- 20.Lau EH, Hsiung CA, Cowling BJ, et al. A comparative epidemiologic analysis of SARS in Hong Kong, Beijing and Taiwan. BMC Infect Dis. 2010;10:50. doi: 10.1186/1471-2334-10-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peiris JS, Cowling BJ, Wu JT, et al. Interventions to reduce zoonotic and pandemic risks from avian influenza in Asia. Lancet Infect Dis. 2016;16(2):252–8. doi: 10.1016/S1473-3099(15)00502-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang L, Cowling BJ, Wu P, et al. Human exposure to live poultry and psychological and behavioral responses to influenza A(H7N9), China. Emerg Infect Dis. 2014;20(8):1296–305. doi: 10.3201/eid2008.131821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X, Wang Q, Cheng W, et al. Risk factors for avian influenza virus contamination of live poultry markets in Zhejiang, China during the 2015-2016 human influenza season. Sci Rep. 2017;7:42722. doi: 10.1038/srep42722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu H, Wu JT, Cowling BJ, et al. Effect of closure of live poultry markets on poultry-to-person transmission of avian influenza A H7N9 virus: an ecological study. Lancet. 2014;383(9916):541–8. doi: 10.1016/S0140-6736(13)61904-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu P, Jiang H, Wu JT, et al. Poultry market closures and human infection with influenza A(H7N9) virus, China, 2013-14. Emerg Infect Dis. 2014;20(11):1891–4. doi: 10.3201/eid2011.140556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gilbert M, Golding N, Zhou H, et al. Predicting the risk of avian influenza A H7N9 infection in live-poultry markets across Asia. Nat Commun. 2014;5:4116. doi: 10.1038/ncomms5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelly H, Cowling BJ. Case fatality: rate, ratio, or risk? Epidemiology. 2013;24(4):622–3. doi: 10.1097/EDE.0b013e318296c2b6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


