It is not unusual for patients who say they are sick to have normal results on standard laboratory testing. The physician often concludes that there is no “real” illness and that the patients’ symptoms likely stem from a psychological disorder. An alternative conclusion, often honored in the breach, is that the standard laboratory tests are measuring the wrong things.
Chronic fatigue syndrome (CFS)―also called myalgic encephalomyelitis/chronic fatigue syndrome―is such an illness. Often, the condition begins suddenly, following an “infectious-like” illness. For years, patients do not return to full health. The illness waxes and wanes, and at its worst leads patients to be bedridden or unable to leave their homes. A report from the National Academies estimates that CFS affects up to 2.5 million people in the United States and generates direct and indirect expenses of $17–24 billion annually (1). The most widely used case definition (2) consists only of symptoms. This, along with typically normal results on standard laboratory tests, has raised the question of whether there are any “real” objective, biological abnormalities in CFS. In PNAS, Montoya et al. (3) report the latest evidence that there are such abnormalities.
Indeed, research over the past 30 y has discovered pathology involving the central nervous system (CNS) and autonomic nervous system (ANS), energy metabolism (with associated oxidative and nitrosative stress), and the immune system, as described in a detailed review (4). This Commentary will briefly summarize the evidence, providing citations only to work published since this review. I will then place the report by Montoya et al. (3) in context, and speculate about the pathophysiology of the illness.
Studies of the Nervous System
Formal testing reveals that CFS patients are more sensitive to painful stimuli, and have cognitive deficits in attention, memory, and reaction time. Spinal fluid studies find elevated numbers of white blood cells and levels of protein, and higher levels of lactic acid, in comparison with both healthy control subjects and patients with neurotic disorders. Spinal fluid contains increased levels of a group of proteins involved in CNS tissue injury and repair.
Standard MRI has repeatedly found abnormalities in the white matter that increase with time and that correlate with symptom scores in CFS patients. Functional MRI has identified unusual brain responses following cognitive, motor, visual, and auditory challenges. Single-photon emission computed tomography has demonstrated hypoperfusion. PET has revealed fewer serotonin transporters in the hippocampus. A small study using PET found evidence of widespread activation of the brain’s innate immune system (5).
Spectral analysis of EEG data produces a pattern that distinguishes patients with CFS from both healthy controls and from patients suffering from major depression. EEG and other technologies also find abnormal “connectivity” between different brain regions.
Neuroendocrine studies demonstrate abnormalities of the hypothalamic–pituitary–adrenal axis, of growth hormone secretion, and of adrenergic metabolism in CFS patients that are distinct from those seen in healthy control subjects and in patients with major depression.
Finally, ANS studies have found strong evidence of disordered sympathetic activity, impaired baroreflex function, exaggerated venous pooling, diminished red cell mass, and reduced plasma volume.
Studies of Energy Metabolism, Oxidative and Nitrosative Stress
Recent metabolomics studies have found deficits in the pathways that generate energy from simple sugars, fatty acids, and amino acids (6–9). Indeed, one study of over 600 metabolites, published in PNAS, found that the levels of most were unusually low, as occurs in states of hibernation (6).
Increased lactate levels in cerebrospinal fluid may indicate impaired oxidative phosphorylation, with a consequent shift to anaerobic metabolism. Possible causes of acquired mitochondrial dysfunction in the CNS include infection and cerebral hypoperfusion.
Many studies have found evidence of oxidative stress in CFS patients: increased levels of isoprostanes, peroxides and superoxide (that correlate with the severity of symptoms), reduced levels of antioxidants (including α-tochopherol), and impaired redox status as reflected in plasma levels of thiobarbituric acid reactive substances (which are correlated with the severity of symptoms) (10). Increased nitrosative stress has also been documented by increased levels of inducible nitric oxide synthase, nitric oxide, peroxynitrite, and nitrate.
Studies of the Immune System
Previously reported immunologic abnormalities in CFS patients include: (i) impaired function of natural killer cells; (ii) increased numbers of activated CD8+ cytotoxic T cells; (iii) the presence of various autoantibodies, particularly to targets in the CNS (11); and (iv) an increased production of various proinflammatory cytokines. The cytokine findings are of interest because cytokines can produce symptoms characteristic of CFS: fatigue, fevers, adenopathy, myalgias, arthralgias, sleep disorders, cognitive impairment, and mood disorders.
So the symptoms of CFS could be produced by the previously reported abnormal cytokine levels, but are they? Or are the abnormal levels merely an epiphenomenon of the illness? That is the central question addressed by the report from Montoya et al. (3). The investigators measured blood levels of 51 “cytokines” (a term that included chemokines and adipokines) in 192 patients with CFS and 392 healthy control subjects matched for age and gender. The severity of the illness in each patient was assessed with a validated instrument, and the patients grouped into “mild,” “moderate,” and “severe” categories. For 17 of the 51 cytokines there was a statistically significant upward linear correlation between the level of the molecule and the severity of the illness, suggesting that the cytokine levels are, indeed, causally associated with the symptoms.
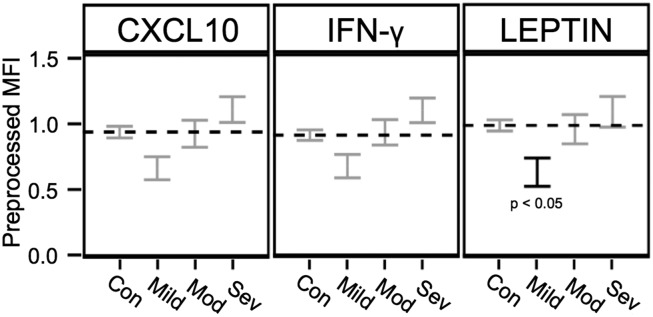
Curiously, of the cytokines linked to illness severity, the levels in the least-severely ill patients were generally lower than those found in healthy control subjects, although the difference was significant only for leptin (3) (Fig. 1). One possibility is that, after several years of an inflammatory illness, the immune system’s capacity to generate high levels of certain cytokines becomes exhausted.
Fig. 1.
Levels of three different immune-related molecules in healthy control subjects and in patients with CFS of different levels of severity: mild, moderate (Mod), and severe (Sev). Levels for three different molecules, CXCL10 (a chemokine), IFN-γ (a cytokine), and leptin (an adipokine) are shown as preprocessed median fluorescence intensity (MFI) ±1 SE. Results were adjusted for multiple comparisons, age, sex, race, and nonspecific binding. The horizontal dashed line is the level for each molecule found in healthy control subjects (Con). For 17 of the 51 immune-related molecules reported by Montoya et al. (3), there was a statistically significant upward trend with severity. This figure is adapted from figure 3 of Montoya et al. (3).
Another large recent study (298 CFS patients, 348 healthy controls), using the same multiplex cytokine assay, suggests just this. Hornig et al. (12) compared results in patients who had been ill for 3 y or less (short term) to those ill longer than 3 y (longer term). Production of 28 of the 51 cytokines was up-regulated in the short-term group, but in the longer-term group levels were normal or lower than normal. It appeared that an initial explosion of cytokine production in the short term was followed by a depletion of cytokine production in the longer term. Montoya et al.’s (3) patient group contained too few short-term patients to allow a robust assessment of this temporal relationship. In any event, the reports of Montoya et al. and others suggest that antiinflammatory therapies may be worth evaluating in CFS.
Speculation as to the Pathophysiology of CFS
If the symptoms of CFS reflect a low-grade inflammation in the CNS, what might be triggering that inflammation? Likely, there are multiple triggers. Possibly, some of the autoantibodies that target cells in the CNS of patients with CFS trigger an inflammatory response (11).
Another possibility is infection, both inside and outside the brain. Many patients state that their illness began with an infectious-like syndrome. Moreover, multiple “postinfectious” fatigue syndromes have been reported since the early 20th century, following either a nondescript infectious-like illness or well-documented infection. The best example of the latter is a prospective observational study of 253 cases of documented acute infections in which 12% of patients developed CFS in the first year following the acute illness (13).
The symptoms of CFS could be produced by the previously reported abnormal cytokine levels, but are they? Or are the abnormal levels merely an epiphenomenon of the illness? That is the central question addressed by the report from Montoya et al.
Patients with severe symptoms and increased production of proinflammatory cytokines during the initial illness were the most likely to develop CFS. In contrast, CFS was not more likely to occur in those with premorbid psychological problems.
It is extremely unlikely that a single, novel infectious agent causes CFS. Indeed, one such claim (involving murine retroviruses) has been refuted (14). Moreover, different infectious agents are capable of triggering (and possibly perpetuating) the illness (4). Several are neurotropic and capable of evading elimination by the immune system. Theoretically, the chronic presence of these agents in the CNS could serve as a goad, eliciting an ineffective and therefore constant low-grade immune attack (4).
In addition, infection outside the brain can activate the innate immune system of the brain, both via a blood–brain barrier made porous by inflammation and via chemoreceptors that send retrograde signals up the vagus nerve to the brain (15).
Another way of incriminating infection in CFS involves the gut microbiome. Several recent studies have reported dysbiosis accompanied by low-grade inflammation and increased permeability of the gut mucosa. This allows bacterial lipopolysaccharide to enter the circulation, possibly activating innate immunity both systemically and in the brain (5, 16–18).
Concluding Thoughts
US President Harry S. Truman famously complained that the economists advising him always said “On the one hand…but on the other hand,” and shouted in exasperation: “Give me a one-handed economist!” President Truman would not have been happy with what follows.
On one hand, it now is clear that there are objective abnormalities in patients with CFS: abnormalities that standard laboratory tests do not measure. On the other hand, not all of these reported abnormalities have yet been repeatedly confirmed. Moreover, even in the largest studies, with the most persuasive evidence of pathology, a substantial minority of patients with CFS do not demonstrate the pathology. On one hand, it is clear that the underlying pathology involves the nervous system, energy metabolism, and the immune system. On the other hand, it is not clear what ties together the pathology seen in these different systems, nor which of the abnormalities came first, nor what triggered that first abnormality. There is much to learn.
Fortunately, the National Institutes of Health has announced it intends to increase its intramural and extramural investment in CFS research, and many laboratories outside the United States are also actively investigating the illness. Hopefully, a decade from now, doctors will know better what to measure and, more importantly, what to do to ease the suffering caused by this illness.
Acknowledgments
The author thanks Emily M. Becker for preparation of the figure, and the Hutchins Family Foundation for support.
Footnotes
The author declares no conflict of interest.
See companion article on page E7150.
References
- 1.Institute of Medicine . Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness. National Academies Press; Washington, DC: 2015. [PubMed] [Google Scholar]
- 2.Fukuda K, et al. International Chronic Fatigue Syndrome Study Group The chronic fatigue syndrome: A comprehensive approach to its definition and study. Ann Intern Med. 1994;121:953–959. doi: 10.7326/0003-4819-121-12-199412150-00009. [DOI] [PubMed] [Google Scholar]
- 3.Montoya JG, et al. Cytokine signature associated with disease severity in chronic fatigue syndrome patients. Proc Natl Acad Sci USA. 2017;114:E7150–E7158. doi: 10.1073/pnas.1710519114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Komaroff AL, Cho TA. Role of infection and neurologic dysfunction in chronic fatigue syndrome. Semin Neurol. 2011;31:325–337. doi: 10.1055/s-0031-1287654. [DOI] [PubMed] [Google Scholar]
- 5.Nakatomi Y, et al. Neuroinflammation in patients with chronic fatigue syndrome/myalgic encephalomyelitis: An 11C-(R)-PK11195 PET study. J Nucl Med. 2014;55:945–950. doi: 10.2967/jnumed.113.131045. [DOI] [PubMed] [Google Scholar]
- 6.Naviaux RK, et al. Metabolic features of chronic fatigue syndrome. Proc Natl Acad Sci USA. 2016;113:E5472–E5480. doi: 10.1073/pnas.1607571113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamano E, et al. Index markers of chronic fatigue syndrome with dysfunction of TCA and urea cycles. Sci Rep. 2016;6:34990. doi: 10.1038/srep34990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fluge Ø, et al. Metabolic profiling indicates impaired pyruvate dehydrogenase function in myalgic encephalopathy/chronic fatigue syndrome. JCI Insight. 2016;1:e89376. doi: 10.1172/jci.insight.89376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Germain A, Ruppert D, Levine SM, Hanson MR. Metabolic profiling of a myalgic encephalomyelitis/chronic fatigue syndrome discovery cohort reveals disturbances in fatty acid and lipid metabolism. Mol Biosyst. 2017;13:371–379. doi: 10.1039/c6mb00600k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fenouillet E, et al. Association of biomarkers with health-related quality of life and history of stressors in myalgic encephalomyelitis/chronic fatigue syndrome patients. J Transl Med. 2016;14:251. doi: 10.1186/s12967-016-1010-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loebel M, et al. Antibodies to β adrenergic and muscarinic cholinergic receptors in patients with chronic fatigue syndrome. Brain Behav Immun. 2016;52:32–39. doi: 10.1016/j.bbi.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 12.Hornig M, et al. Distinct plasma immune signatures in ME/CFS are present early in the course of illness. Sci Adv. 2015;1:e1400121. doi: 10.1126/sciadv.1400121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hickie I, et al. Dubbo Infection Outcomes Study Group Post-infective and chronic fatigue syndromes precipitated by viral and non-viral pathogens: Prospective cohort study. BMJ. 2006;333:575–578. doi: 10.1136/bmj.38933.585764.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simmons G, et al. Blood XMRV Scientific Research Working Group (SRWG) Failure to confirm XMRV/MLVs in the blood of patients with chronic fatigue syndrome: A multi-laboratory study. Science. 2011;334:814–817. doi: 10.1126/science.1213841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poon DC, Ho YS, Chiu K, Wong HL, Chang RC. Sickness: From the focus on cytokines, prostaglandins, and complement factors to the perspectives of neurons. Neurosci Biobehav Rev. 2015;57:30–45. doi: 10.1016/j.neubiorev.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 16.Nagy-Szakal D, et al. Fecal metagenomic profiles in subgroups of patients with myalgic encephalomyelitis/chronic fatigue syndrome. Microbiome. 2017;5:44. doi: 10.1186/s40168-017-0261-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maes M, et al. Increased IgA responses to the LPS of commensal bacteria is associated with inflammation and activation of cell-mediated immunity in chronic fatigue syndrome. J Affect Disord. 2012;136:909–917. doi: 10.1016/j.jad.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 18.Giloteaux L, et al. Reduced diversity and altered composition of the gut microbiome in individuals with myalgic encephalomyelitis/chronic fatigue syndrome. Microbiome. 2016;4:30. doi: 10.1186/s40168-016-0171-4. [DOI] [PMC free article] [PubMed] [Google Scholar]