Abstract
Background
Adjunctive corticosteroids therapy is an attractive option for community-acquired pneumonia (CAP) treatment. However, the effectiveness of adjunctive corticosteroids on mortality of CAP remains inconsistent, especially in severe CAP. We performed a meta-analysis to evaluate the efficacy and safety of adjunctive corticosteroids in severe CAP patients.
Methods
Three databases of PubMed, EMBASE and Cochrane Library were searched for related studies published in English up to December, 2015. Randomized controlled trials (RCTs) of corticosteroids in hospitalized adults with severe CAP were included. Meta-analysis was performed by a random-effect model with STATA 11.0 software. We estimated the summary risk ratios (RRs) or effect size (ES) with its corresponding 95% confidence interval (95%CI) to assess the outcomes.
Results
We included 8 RCTs enrolling 528 severe CAP patients. Adjunctive corticosteroids significantly reduced all-cause mortality (RR = 0.46, 95%CI: 0.28 to 0.77, p = 0.003), risk of adult respiratory distress syndrome (ARDS) (RR = 0.23, 95%CI: 0.07 to 0.80, p = 0.02) and need for mechanical ventilation (RR = 0.50, 95%CI: 0.27 to 0.92, p = 0.026). Adjunctive corticosteroids did not increase frequency of hyperglycemia requiring treatment (RR = 1.03, 95%CI: 0.61 to 1.72, p = 0.91) or gastrointestinal hemorrhage (RR = 0.66, 95%CI: 0.19 to 2.31, p = 0.52). In subgroup analysis by duration of corticosteroids, we found that prolonged corticosteroids therapy significantly reduced all-cause mortality (RR = 0.41, 95%CI: 0.20 to 0.83, p = 0.01) and length of hospital stay (−4.76 days, 95% CI:-8.13 to -1.40, p = 0.006).
Conclusions
Results from this meta-analysis suggested that adjunctive corticosteroids therapy was safe and beneficial for severe CAP. In addition, prolonged corticosteroids therapy was more effective. These results should be confirmed by adequately powered studies in the future.
Introduction
Community-acquired pneumonia (CAP) is a common and serious infectious disease accompanied with high morbidity and mortality [1], about 20% of CAP adult patients acquire hospitalization, and has a mortality of 30–50% [2]. Furthermore, severe CAP occurs in approximately 10% of hospitalized patients with CAP, and has a higher mortality [3]. Despite recent advances in antimicrobial therapy and life-support measures, the mortality of severe CAP has not declined yet [4, 5]. Therefore, potential therapies of severe CAP should be explored.
Pneumonia is characterized by lung inflammation, with fluid filling the alveoli and preventing adequate oxygenation of the body [6]. During infectious pneumonia, an excessive release of circulating inflammatory cytokines, such as interleukin (IL)-10, IL-8 and IL-6 that acted as acute phase markers, would cause pulmonary dysfunction [7]. A recent study demonstrated that the raised levels of IL-6 and IL-10 were related with a high mortality in CAP, especially in severe CAP [8] which could potentially increase the incidences of sepsis, lung injury and acute respiratory distress syndrome (ARDS) [9]. Therefore, active and effective prevention of inflammatory deterioration is vital to severe CAP treatment.
Currently, corticosteroids are the most potent anti-inflammatory drugs. The therapeutic mechanism of corticosteroids might base on attenuating the action of many cytokines that participated in the inflammatory reaction associating severe CAP [10]. In addition, with the proposition of critical illness-related corticosteroid insufficiency (CIRCI), corticosteroids replacement therapy has been gradually accepted in critical illness, such as septic shock and ARDS [11]. Salluh et al. found that the most of patients with severe CAP could have relative adrenal insufficiency [12]. Another study also demonstrated that the reduction of baseline cortisol level could excerbate the severity and outcomes of severe CAP [13]. Up to date, many physicians have been using corticosteroids for severe CAP patients though the best usage regimens of corticosteroids were unclear [14]. Taken together, these facts indicated corticosteroids were of potential benefit in severe CAP.
In recent decades, adjunctive corticosteroids in severe CAP has been assessed in many randomized controlled trials (RCTs) [15–28]. However, the results of previous RCTs were inconsistent, and meta-analyses conducted to evaluate those RCTs had also failed to establish a complete agreement [29–37]. Recent meta-analyses conducted by Horita et al. [35] and Siemieniuk et al. [36] revealed that adjunctive corticosteroids statistically reduced the mortality of severe CAP. However, Wan et al. [37] proved that adjunctive corticosteroids was not associated with decreased mortality of patients with severe CAP. Therefore, we believed a meta-analysis, which depending on the best updating available evidence, was required. The aim of this study was to evaluate the efficacy and safety of adjunctive corticosteroids in the management of adult hospitalized patients with severe CAP.
Materials and Methods
This study was conducted adhering to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.
Search strategy
We conducted a literature search in PubMed, EMBASE and Cochrane Library up to December, 2015. The search strategy included the following key words: “(severe community acquired pneumonia OR severe CAP) AND (corticosteroids OR corticotherapy OR steroids OR dexamethasone OR methylprednisolone OR prednisone OR cortisone OR hydrocortisone OR prednisolone)”. The search was limited to studies written in English and those utilizing RCT subjects. The detailed search strategy was available in S3 File. To ensure a comprehensive literature search, we also examined reference lists from included articles.
Selection criteria
In this meta-analysis, we included studies that met the following eligibility criteria: (1) population: aged 18 years or older; (2) study design: RCTs (including quasi-RCTs), English language; (3) intervention: corticosteroids adjunctive therapy in severe CAP; (4) control intervention: placebo or standard treatment; (5) any types, any doses, and any durations of systemic corticosteroids were allowed; (6) primary outcome: all-cause mortality; (7) secondary outcomes: length of ICU stay, length of hospital stay, ARDS, need for mechanical ventilation and adverse effects. Articles were excluded based on the following criteria: (1) studies only had abstracts; (2) studies enrolled pediatric patients, nosocomial pneumonia patients or specified pathogens.
Data Abstraction
Two reviewers extracted data independently and resolved disagreements by a consensus. Data was extracted from those included studies according to the pre-designed form: first author, year of publication, study design, location, number of patients, participant demographics, duration of corticosteroids treatment, doses of corticosteroids, evaluation criteria of severe CAP and outcomes. We contacted the authors of the included studies by E-mail if further study details were needed.
In previous meta-analyses, severe CAP was defined according to common criteria: CURB-65 (confusion, urea nitrogen, respiratory rate, blood pressure, and age 65 years or older) score of 2 or greater [38], Pneumonia Severity Index score (PSI) of IV or V [39], the American Thoracic Society (ATS) [40], and the British Thoracic Society criteria (BTS) [41]. The four proxies for “severe” CAP have advantages and disadvantages in clinical practice [42]. In addition, the two prediction tools SMART-COP [43] and predisposition, insult, response, and organ dysfunction (PIRO) [44] which are required further validation have similar sensitivity and specificity with ATS or outperform ATS [45]. In this study, we selected severe cases depended on the above tools, authors' classification of severe CAP when objective scoring was not available and limited to ICU cases.
Data Synthesis and Statistical Analysis
If both per-protocol-analysis and intention-to-treat (ITT) analysis were conducted, ITT manner was chosen. Throughout the meta-analysis, we calculated risk ratios (RRs) and 95% confidence interval (CI) across all studies using a Mantel-Haenszel random-effects model for dichotomous outcomes and the effect size (ES) using an inverse-variance random effects model [46]. Heterogeneity was evaluated using the I2 test statistic and classified as no heterogeneity (I2 = 0), the least (≤25%), mild (25–50%), moderate (50–75%), and strong (≥75%) [47]. A sensitivity analysis was implemented by the sequential dropping of each study. The potential publication bias was assessed via funnel plots [48]. A p<0.05 was considered statistically significant. All statistical analyses were undertaken in STATA 11.0 software (Stata Corporation, College Station, TX).
Results
Trial flow
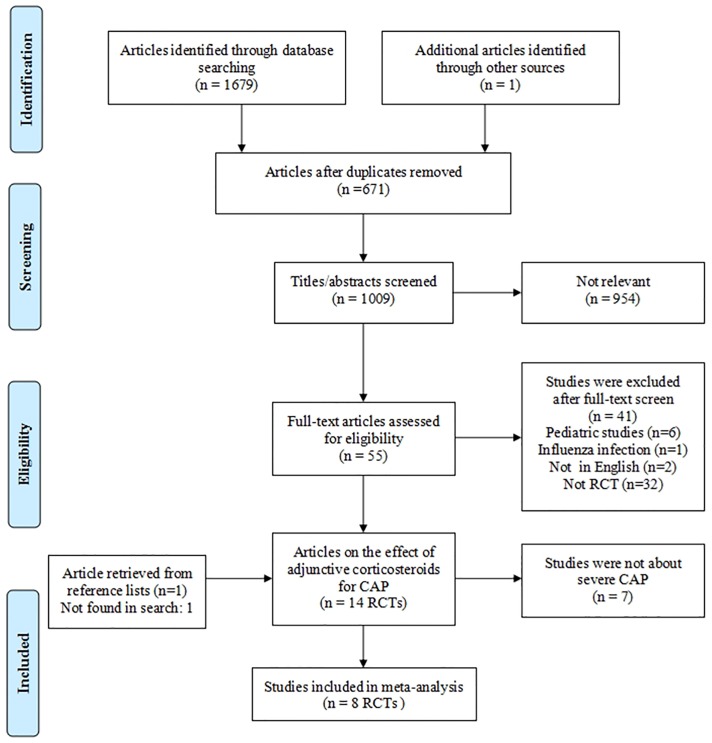
Our study design produced 1679 studies. Based on the inclusion and exclusion criteria, 1672 studies were excluded. Finally, 8 RCTs were included for meta-analysis [17–19, 21, 22, 24, 26, 28], and a PRISMA flow diagram of selection process for studies was shown in Fig 1. Among included RCTs, one study included mild to severe CAP patients, we selected the severe CAP cases which were defined by PSI of IV or V[21]. A study by El-Ghamrawy [19] which was not found in Search Databases, we obtained data from previous review [36].
Fig 1. Flow diagram of the study selection process.
Study characteristics
Table 1 described the main characteristics of inculded studies, including 286 patients in the corticosteroids group and 242 patients in the control group. The different types of corticosteroids including hydrocortisone, methyl-prednisolone, dexamethasone and prednisolone. The durations of corticosteroids treatment ranged from 1 to 9 days. Various scoring systems, authors' classification of severe CAP and ICU cases were used to define severe CAP. Detailed extractive information from included studies were presented in Tables 1 and 2.
Table 1. Characteristics of included randomized controlled trials in the meta-analysis.
| Study, year | Location | Study design | Sample sizes | Mean age (y) | Severe criterion | Corticosteroids used | Outcome | |
|---|---|---|---|---|---|---|---|---|
| n | N | |||||||
| Marik [17], 1993 | USA | DB RCT | 14 | 16 | 34 | ICU, BTS | Hydrocortisone, 10 mg/kg, 1d | Mortality |
| Confalonieri [18], 2005 | Italy | DB RCT | 23 | 23 | 64 | ICU, ATS | Hydrocortisone, 240 mg/d, 7d | Hospital mortality |
| El-Ghamray [19], 2006 | Saudi Arabia | DB RCT | 17 | 17 | 61.8 | ICU, ATS | Hydrocortisone 200 mg IV bolus followed by 10 mg/h for 7d | Hospital mortality |
| Snijders [21], 2010 | Netherlands | DB RCT | 48 | 45 | 63 | PSI | Prednisolone, 40 mg/d, 7d | 30-d mortality |
| Sabry [24], 2011 | Egypt | DB RCT | 40 | 40 | 62 | ATS | Hydrocortisone, 300mg/d, 7d | ICU mortality |
| Fernandez-Serrano [22], 2011 | Spain | DB RCT | 23 | 22 | 63 | PSI | Methylprednisolone, 620mg, 9d | Mortality |
| Nafae [26], 2013 | Egypt | Open-label RCT | 60 | 20 | 49 | PSI | Hydrocortisone, 200mg (at day 1) and the 10mg/h IV for 7d | Mortality |
| Torres [28], 2015 | Spain | DB RCT | 61 | 59 | 65.3 | ICU, ATS | Methylprednisolone, 0.5mg/Kg, 2/d, 5d | Hospital mortality |
DB: double-blind; RCT: randomized controlled trial; ATS: American Thoracic Society; BTS: British Thoracic Society; PSI: Pneumonia Severity Index; ICU: intensive care unit; IV: intravenous. Severe community-acquired pneumonia is defined as PSI of IV or V, CURB-65 score ≥2; Meeting 1 of the ATS 1993 criteria; ATS 2001 rule where 1 major or 2 minor criteria are satisfied; ATS-IDSA 2007 rule where 1 major or 3 minor criteria are satisfied; BTS≥3. n: the number of patients in the corticosteroids group, N: the number of patients in the control group.
Table 2. Outcome data per study.
| Study,Year | Sample size (n/N) | Mortality (n/N) | Treatment duration (n/N) | Length of hospital stay, d (n/N) | Length of ICU stay, d (n/N) | Developed ARDS (n/N) | Required mechanical ventilation (n/N) | Hyperglycemia requiring treatment (n/N) | Gastrointestinal bleeding (n/N) |
|---|---|---|---|---|---|---|---|---|---|
| Marik, 1993 | 14/16 | 1/3 | ≤5d | - | 4.30±3.80/4.60±5.90 | - | 2/4 | - | - |
| Confalonieri, 2005 | 23/23 | 0/8 | >5d | 22.25±10.75/29.25±17.25 | 14.25±7.25/21.00±10.25 | 0/4 | - | - | 1/1 |
| El-Ghamray, 2006 | 17/17 | 3/6 | >5d | - | - | 0/3 | - | 1/1 | |
| Snijders, 2010 | 48/45 | 4/3 | >5d | - | - | - | - | - | |
| Sabry, 2011 | 40/40 | 2/6 | >5d | - | - | 2/6 | - | - | |
| Fernandez-Serrano, 2011 | 23/22 | 1/1 | >5d | 10.00±2.96/12±6.67 | 6.50±2.69/10.50±13.52 | - | 1/5 | 1/0 | 1/1 |
| Nafae, 2013 | 60/20 | 4/6 | >5d | 9.27±2.40/16.50±2.24 | 3.10±4.90/6.30±8.20 | - | 8/5 | 19/8 | 1/1 |
| Torres, 2015 | 61/59 | 6/9 | ≤5d | 11.00±4.81/10.50±5.19 | 5.00±3.70/6.00±2.96 | - | 5/9 | 11/7 | 0/1 |
ICU: intensive care unit; ARDS: acute respiratory distress syndrome; n: the number of patients in the corticosteroids group, N: the number of patients in the control group.
Quality Assessment
Two authors (Jirui Bi and Jin Yang) carried out data extraction and assessed the risk of bias independently, any conflicts were resolved with group consensus. The Cochrane Collaboration’s Risk of Bias Tool [49] was used to assess the quality of all included RCTs (Table 3).
Table 3. Summarizes the risk of bias.
| Author | Randomization method | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other bias | Overall risk |
|---|---|---|---|---|---|---|---|---|
| Marik | Low | Low | Low | Low | Low | Low | Unclear | Low |
| Confalonieri | Low | Low | Low | Low | Low | Low | Unclear | Low |
| El-Ghamray | Low | Unclear | Unclear | Low | Low | Low | Unclear | Low |
| Snijders | Low | Low | Low | Low | Low | Low | Low | Low |
| Sabry | Low | Low | Low | Low | Low | Low | Unclear | Low |
| Fernandez-Serrano | Low | Unclear | Low | Low | Unclear | Low | Low | Low |
| Nafae | Unclear | Low | Unclear | Unclear | Unclear | Low | Unclear | High |
| Torres | Low | Unclear | Low | Low | Low | Low | Low | Low |
Primary Outcome
The main results for binary outcomes (Mantel-Haenszel method, random effect model) were presented in Table 4.
Table 4. Main results for binary outcomes (Mantel-Haenszel method, random effect model).
| Outcome | Studies, n | Pooled RR (or ES) (95% CI) | p-value | I2, % | pheterogenelty |
|---|---|---|---|---|---|
| All-cause mortality | 8 | 0.46 (0.28 to 0.77) | 0.003 | 0.0 | 0.481 |
| Prescription duration | |||||
| ≤5d | 2 | 0.59 (0.24 to 1.43) | 0.241 | 0.0 | 0.661 |
| >5d | 6 | 0.41 (0.21 to 0.82) | 0.012 | 15.1 | 0.317 |
| Length of hospital stay | 3 | -4.76 (-8.13 to -1.40) | 0.006 | 43.4 | 0.170 |
| Length of ICU stay | 5 | -1.84 (-4.23 to 0.56) | 0.130 | 38.6 | 0.182 |
| Developed ARDS | 3 | 0.23 (0.07 to 0.80) | 0.020 | 0.0 | 0.743 |
| Required mechanical ventilation | 4 | 0.50 (0.27 to 0.92) | 0.026 | 0.0 | 0.841 |
| Adverse effects | |||||
| hyperglycemia | 3 | 1.03 (0.61 to 1.72) | 0.91 | 0.0 | 0.385 |
| gastrointestinal hemorrhage | 5 | 0.66 (0.19 to 2.31) | 0.52 | 0.0 | 0.954 |
RR: risk ratio; ES: effect size; ICU: intensive care unit; ARDS: acute respiratory distress syndrome.
All-Cause Mortality
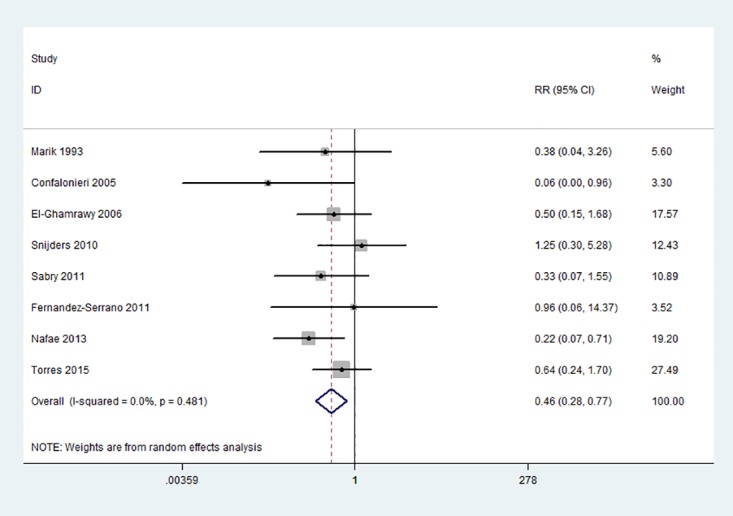
The pooled results of all-cause mortality was significantly reduced by the use of corticosteroids (8 trials, RR = 0.46, 95%CI: 0.28 to 0.77, p = 0.003, I2 = 0%) (Fig 2).
Fig 2. Forest plots of the association between all-cause mortality of severe CAP and corticosteroids.
Subgroup analysis was performed by duration of corticosteroids treatment. Corticosteroids therapy reduced all-cause mortality in severe CAP, but the best corticosteroids duration for severe CAP was not fully clear. Our analysis suggested that prolonged use of corticosteroids (>5 days) was more effective for severe CAP (RR = 0.41, 95%CI: 0.21 to 0.82, p = 0.01; I2 = 15.1%) (Fig 3). There was no statistically significant difference in short course of corticosteroids (≤5 days) (2 trials; RR = 0.59, 95%CI: 0.24 to 1.43, p = 0.241; I2 = 0%).
Fig 3. Subgroup analysis according to the duration of corticosteroids treatment in severe CAP.

Secondary outcomes
Length of hospital stay
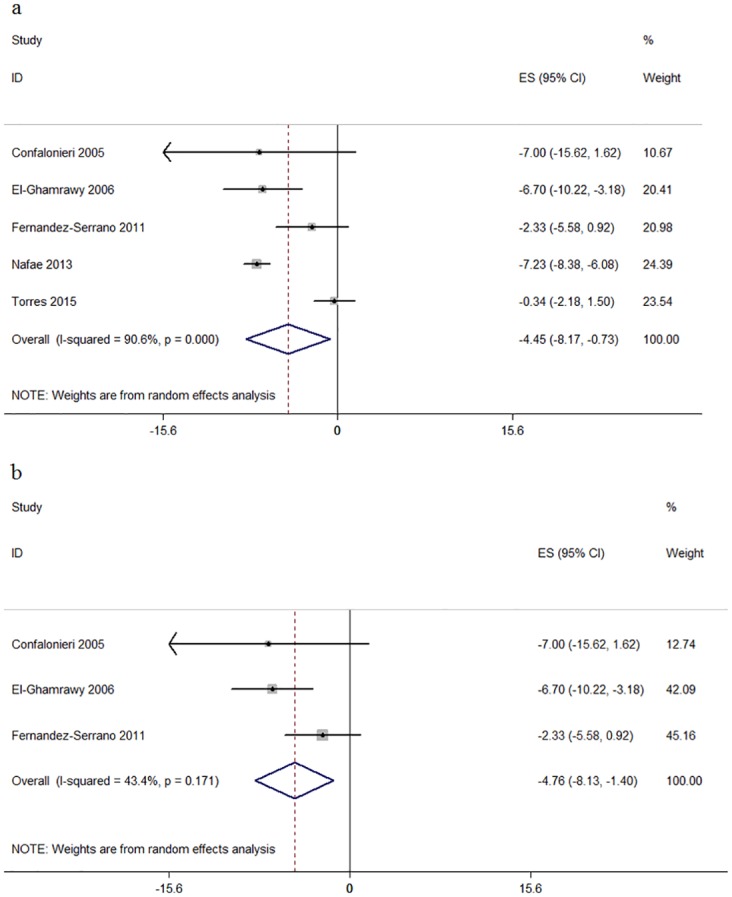
5 RCTs reported the difference of length of hospital stay in days. Based on these data, we found strong heterogeneity (I2 = 90.6%) in the primary analysis of hospital stay days (Fig 4a). In subgroup analysis by the treatment duration of corticosteroids, the result was −5.79 days (95% CI:-8.17 to -2.31, I2 = 61.4%), there was also moderate heterogeneity. We performed a sensitivity analysis excluding Nafae, and the final result was −4.76 days (95% CI:-8.13 to -1.40, p = 0.006; I2 = 43.4%, p for heterogeneity = 0.17) (Fig 4b).
Fig 4. Forest plots of the association between length of hospital stay and corticosteroids.
Length of ICU stay
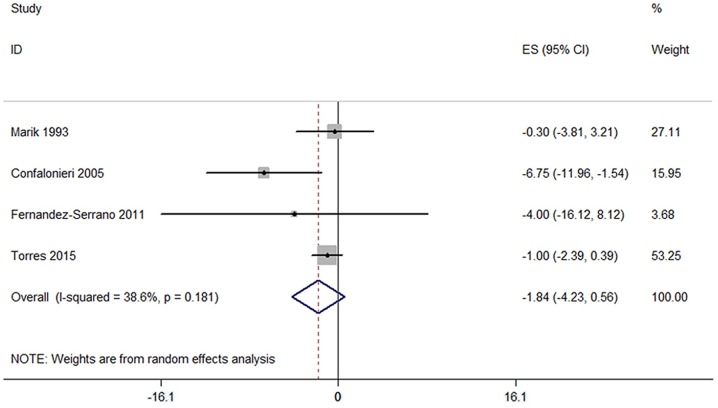
Based on the data of 4 RCTs, we found that corticosteroids did not significantly decrease the length of ICU stay (−1.84 days, 95% CI:-4.23 to 0.56, p = 0.13, I2 = 38.6%) (Fig 5).
Fig 5. Forest plots of the association between length of ICU stay and corticosteroids.
Acute Respiratory Distress Syndrome
3 RCTs evaluated the risk of ARDS in patients. We found a statistically significant reduction in the risk of ARDS treated with corticosteroids (RR = 0.23, 95%CI: 0.07 to 0.80, p = 0.02; I2 = 0%) (Fig 6).
Fig 6. Effect of corticosteroids on development of ARDS and need for mechanical ventilation in patients hospitalized with severe CAP.
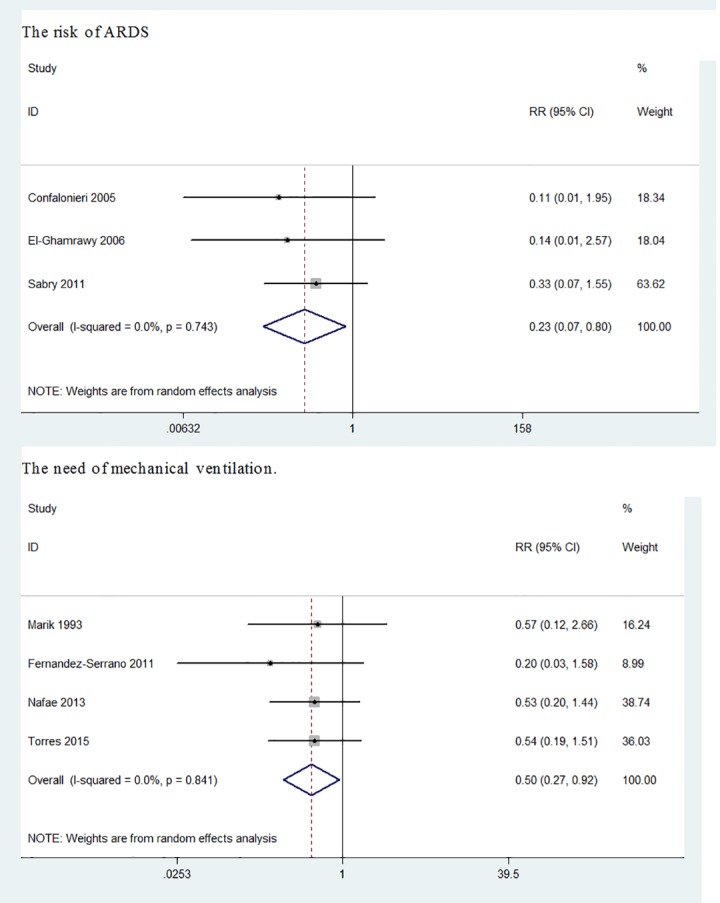
Mechanical ventilation
4 RCTs revealed a reduction in the need for mechanical ventilation in patients treated with corticosteroids (RR = 0.50, 95%CI: 0.27 to 0.92, p = 0.026; I2 = 0%) (Fig 6).
Adverse effects
The adverse effects of corticosteroids therapy mainly included hyperglycemia and gastrointestinal hemorrhage. The results of the analysis showed that systemic corticosteroids had no effects on hyperglycemia (3 trials; RR = 1.03, 95%CI: 0.61 to 1.72, p = 0.91; I2 = 0%) and gastrointestinal hemorrhage (5 trials; RR = 0.66, 95%CI: 0.19 to 2.31, p = 0.52; I2 = 0%) (Fig 7).
Fig 7. Effect of corticosteroids on hyperglycemia and gastrointestinal hemorrhage in severe CAP patients.
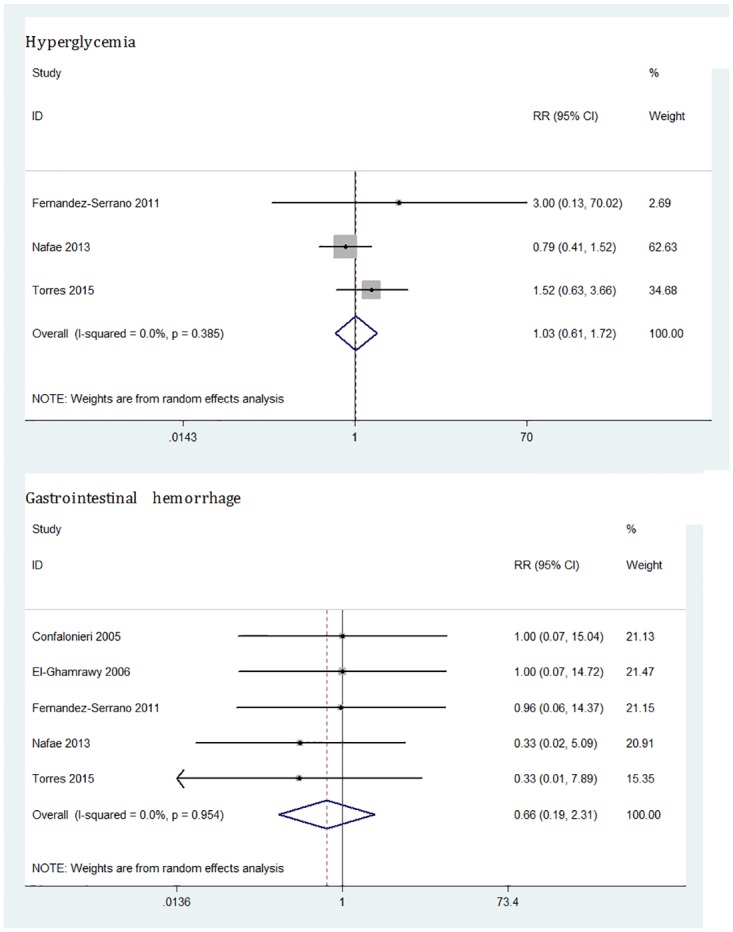
Sensitivity analysis and publication bias
A sensitivity analysis was implemented by the sequential dropping of each study. In above analyses, we just used this analytical method in length of hospital stay.
Considering the limited number (<10) of studies included, we did not evaluate publication bias in this systematic review.
Discussion
This systematic review and meta-analysis had included 8 RCTs, which evaluated the outcomes of adjunctive corticosteroids therapy in adult patients with severe CAP. The main results of meta-analysis were as follows: (1) corticosteroids reduced all-cause mortality for severe CAP; (2) prolonged corticosteroids therapy shortened length of hospital stay for severe CAP; (3) corticosteroids reduced the risk of ARDS and the need for mechanical ventilation. Additionally, our analysis demonstrated that the incidence of hyperglycemia was increased by systemic corticosteroids but the difference was not statistically significant.
In this meta-analysis, the results demonstrated a statistically significant decrease in the mortality of severe CAP patients treated with corticosteroids. We included all eligible trials with high quality and a low risk of bias except one study [26]. Usually, clinicians used adjunctive corticosteroids therapy for severe CAP especially in the early stage. And there are three potential reasons for the survival advantage. First, corticosteroids are the most powerful inhibitor of inflammation which is part of the complex biological reaction to harmful stimuli such as trauma and pathogens. In addition, the pathogenesis of the majority of severe CAP is usually closely related with the excessive inflammatory response [50]. The anti-inflammatory mechanism of corticosteroids is not completely clear; however, it is believed that corticosteroids play an important role in switching on genes that encode anti-inflammatory cytokines and switching off genes that encode pro-inflammatory cytokines [10, 51]. And in a model of Pseudomonas aeruginosa with mechanical ventilation, the results showed that the treatment of antibiotics plus corticosteroids diminished the inflammatory response, decreased bacterial burden in lungs and improved the histopathological severity of pneumonia [52]. Second, the CIRCI was closely associated with severe CAP apart from the excessive inflammation. A systematic review revealed that severe CAP had a prevalence of CIRCI ranging from 0% to 48% [30], and the elevated cortisol levels could be a useful prognostic marker in patients with severe CAP [12]. Therefore, corticosteroids replacement therapy might be effective in severe CAP. Finally, corticosteroids could reduce the mortality of severe CAP for the reason of the reduction in the risk of ARDS and the need for mechanical ventilation [53].
The optimal corticosteroids regimens for severe CAP are not fully explored. The duration of corticosteroids treatment in those studies, which ranged from 1 to 9 days, was five days on average with a median of seven days. Horita et al. found that prolonged use of corticosteroids (>5 days) was not more effective than a short course (≤5 days). To the contrary, our results suggested that prolonged use of corticosteroids (>5 days) was more effective for severe CAP. It is noteworthy that the number of studies refered to the short course of corticosteroids was small (2 studies). Addtionally, The optimal dosage of corticosteroids is also unclear. Most studies used hydrocortisone 200–300mg/day, prednisolone/methylprednisolone 20–50mg/day, or methylprednisolone 1mg/kg/day [35], which were so-called low-or middle-dose of corticosteroids and would be the most suitable dosage in clinical application. Therefore, physicians needed to adjust the dosage and duration of corticosteroids individually.
Our study demonstrated that prolonged use of corticosteroids (>5 days) decreased the length of hospital stay in severe CAP patients. At first, we estimated the result using the value of medians in 5 studies and found a strong heterogeneity (I2 = 90.6%). There were three main sources of heterogeneity: methodological heterogeneity, clinical heterogeneity and statistic heterogeneity [47]. In our study, heterogeneity mainly stemed from methodological heterogeneity and clinical heterogeneity, so we conducted a subgroup analysis and sensitivity analysis. Subgroup analysis performed by duration of corticosteroids, the result was -5.79 days (95%CI: -8.17 to -2.31, I2 = 61.4%). Considering the study of Nafae with a high risk of bias, we performed a sensitivity analysis to exclude Nafae, and the final result was -4.76 days (95%CI: -8.13 to -1.40, I2 = 43.4, p for heterogeneity = 0.17). Adjunctive corticosteroids therapy not only reduced the hospitalization time of severe CAP, but also alleviated the suffering of patients, and finally reduced the financial social-economic burdens [54]. In our study, the potential adverse effects of corticosteroids in severe CAP should be clarified. Theoretically, corticosteroids therapy has the following adverse effects: metabolic disorders (mainly including hyperglycemia), muscle weakness, gastroduodenal bleeding and superinfection. A previous study showed that corticosteroids therapy increased the risks of hypernatremia and hyperglycemia [55]. In our meta-analysis, treatment with corticosteroids in severe CAP was associated with an increased risk of hyperglycemia but the difference had not statistically significant. However, we have not analyzed the association of corticosteroids and the risk of hypernatremia or superinfection, since sufficient information could not be extracted from primary publications. We considered rigorous and comprehensive studies should be designed.
Limitations of this study should be considered: (1) A small number of included studies and relatively small samples had significant influences on the reliability of the results. (2) The definition of severe CAP was not consistent in these studies. It is difficult to achieve a uniform diagnostic criteria due to individual differences in patient, the complex of disease, doctors’ subjective judgments and various severe CAP scoring systems. Therefore, diagnosis of severe CAP in clinical practieces should be comprehensive, included demographic, clinical features plus radiographic, laboratory (some useful biochemical indicators), and microbiologic testing. (3) The opitmal corticosteroids regimens for severe CAP were not fully clarified, which were associated with the differences in type, duration and dosage of corticoteroids, antibiotic therapy, and drug tapering course. (4) The detailed descriptions of severe CAP patients, such as a high inflammatory response, complications, and gender differences, were not fully clear.
In conclusions, our results suggested that adjunctive corticosteroids therapy decreased all-cause mortality in patients with severe CAP, and prolonged corticosteroids treatment was more beneficial for severe CAP. In addition, the study indicated that corticosteroids treatment was safe and reduced the risk of ARDS and the need for mechanical ventilation. Considering the limited number of severe CAP patients, the results may not be stabilized. Thus, large-scale, randomized, double-blind, placebo-controlled trials are needed to evaluate the efficacy and safety of adjunctive corticosteroids therapy in adults with severe CAP.
Supporting Information
(PDF)
(DOCX)
(DOCX)
Acknowledgments
This systematic review and meta-analysis was performed in the supports of Department of Respiratory Medicine, the Second Affiliated Hospital of Anhui Medical University. We would like to express our deepest appreciation for all the members who help us to accomplish this study.
Data Availability
All relevant data are within the paper and its Supporting Informantion files.
Funding Statement
The funders had a role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Woodhead M, Welch CA, Harrison DA, Bellingan G, Ayres JG. Community-acquired pneumonia on the intensive care unit: secondary analysis of 17,869 cases in the ICNARC Case Mix Programme Database. Crit Care. 2006;10 Suppl 2:S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Said MA, Johnson HL, Nonyane BA, Deloria-Knoll M, O'Brien KL, Andreo F, et al. Estimating the burden of pneumococcal pneumonia among adults: a systematic review and meta-analysis of diagnostic techniques. PLoS One. 2013;8(4):e60273 10.1371/journal.pone.0060273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wunderink RG, Waterer GW. Clinical practice. Community-acquired pneumonia. N Engl J Med. 2014;370(6):543–51. 10.1056/NEJMcp1214869 [DOI] [PubMed] [Google Scholar]
- 4.Alvarez-Lerma F, Torres A. Severe community-acquired pneumonia. Curr Opin Crit Care. 2004;10(5):369–74. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez A, Mendia A, Sirvent JM, Barcenilla F, de la Torre-Prados MV, Sole-Violan J, et al. Combination antibiotic therapy improves survival in patients with community-acquired pneumonia and shock. Crit Care Med. 2007;35(6):1493–8. 10.1097/01.CCM.0000266755.75844.05 [DOI] [PubMed] [Google Scholar]
- 6.Mandell LA. Community-acquired pneumonia: An overview. Postgraduate medicine. 2015;127(6):607–15. 10.1080/00325481.2015.1074030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Endeman H, Meijvis SC, Rijkers GT, van Velzen-Blad H, van Moorsel CH, Grutters JC, et al. Systemic cytokine response in patients with community-acquired pneumonia. The European respiratory journal. 2011;37(6):1431–8. 10.1183/09031936.00074410 [DOI] [PubMed] [Google Scholar]
- 8.Martinez R, Menendez R, Reyes S, Polverino E, Cilloniz C, Martinez A, et al. Factors associated with inflammatory cytokine patterns in community-acquired pneumonia. The European respiratory journal. 2011;37(2):393–9. 10.1183/09031936.00040710 [DOI] [PubMed] [Google Scholar]
- 9.Aliberti S, Brambilla AM, Chalmers JD, Cilloniz C, Ramirez J, Bignamini A, et al. Phenotyping community-acquired pneumonia according to the presence of acute respiratory failure and severe sepsis. Respiratory research. 2014;15:27 10.1186/1465-9921-15-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids—new mechanisms for old drugs. N Engl J Med. 2005;353(16):1711–23. 10.1056/NEJMra050541 [DOI] [PubMed] [Google Scholar]
- 11.Marik PE, Pastores SM, Annane D, Meduri GU, Sprung CL, Arlt W, et al. Recommendations for the diagnosis and management of corticosteroid insufficiency in critically ill adult patients: consensus statements from an international task force by the American College of Critical Care Medicine. Crit Care Med. 2008;36(6):1937–49. 10.1097/CCM.0b013e31817603ba [DOI] [PubMed] [Google Scholar]
- 12.Salluh JI, Verdeal JC, Mello GW, Araujo LV, Martins GA, de Sousa Santino M, et al. Cortisol levels in patients with severe community-acquired pneumonia. Intensive Care Med. 2006;32(4):595–8. 10.1007/s00134-005-0046-9 [DOI] [PubMed] [Google Scholar]
- 13.Salluh JI, Bozza FA, Soares M, Verdeal JC, Castro-Faria-Neto HC, Lapa ESJR, et al. Adrenal response in severe community-acquired pneumonia: impact on outcomes and disease severity. Chest. 2008;134(5):947–54. 10.1378/chest.08-1382 [DOI] [PubMed] [Google Scholar]
- 14.Tagami T, Matsui H, Horiguchi H, Fushimi K, Yasunaga H. Low-dose corticosteroid use and mortality in severe community-acquired pneumonia patients. The European respiratory journal. 2015;45(2):463–72. 10.1183/09031936.00081514 [DOI] [PubMed] [Google Scholar]
- 15.Wagner HN Jr., Bennett IL Jr., Lasagna L, Cluff LE, Rosenthal MB, Mirick GS. The effect of hydrocortisone upon the course of pneumococcal pneumonia treated with penicillin. Bull Johns Hopkins Hosp. 1956;98(3):197–215. [PubMed] [Google Scholar]
- 16.McHardy VU, Schonell ME. Ampicillin dosage and use of prednisolone in treatment of pneumonia: co-operative controlled trial. Br Med J. 1972;4(5840):569–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marik P, Kraus P, Sribante J, Havlik I, Lipman J, Johnson DW. Hydrocortisone and tumor necrosis factor in severe community-acquired pneumonia. A randomized controlled study. Chest. 1993;104(2):389–92. [DOI] [PubMed] [Google Scholar]
- 18.Confalonieri M, Urbino R, Potena A, Piattella M, Parigi P, Puccio G, et al. Hydrocortisone infusion for severe community-acquired pneumonia: a preliminary randomized study. Am J Respir Crit Care Med. 2005;171(3):242–8. 10.1164/rccm.200406-808OC [DOI] [PubMed] [Google Scholar]
- 19.El-Ghamrawy AH, Shokeir MH, Esmat AA. Effects of low-dose hydrocortisone in ICU patients with severe community-acquired pneumonia. Egyptian Journal of Chest. 2006;55:91–9. [Google Scholar]
- 20.Mikami K, Suzuki M, Kitagawa H, Kawakami M, Hirota N, Yamaguchi H, et al. Efficacy of corticosteroids in the treatment of community-acquired pneumonia requiring hospitalization. Lung. 2007;185(5):249–55. 10.1007/s00408-007-9020-3 [DOI] [PubMed] [Google Scholar]
- 21.Snijders D, Daniels JM, de Graaff CS, van der Werf TS, Boersma WG. Efficacy of corticosteroids in community-acquired pneumonia: a randomized double-blinded clinical trial. Am J Respir Crit Care Med. 2010;181(9):975–82. 10.1164/rccm.200905-0808OC [DOI] [PubMed] [Google Scholar]
- 22.Fernandez-Serrano S, Dorca J, Garcia-Vidal C, Fernandez-Sabe N, Carratala J, Fernandez-Aguera A, et al. Effect of corticosteroids on the clinical course of community-acquired pneumonia: a randomized controlled trial. Crit Care. 2011;15(2):R96 10.1186/cc10103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meijvis SC, Hardeman H, Remmelts HH, Heijligenberg R, Rijkers GT, van Velzen-Blad H, et al. Dexamethasone and length of hospital stay in patients with community-acquired pneumonia: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;377(9782):2023–30. 10.1016/S0140-6736(11)60607-7 [DOI] [PubMed] [Google Scholar]
- 24.Sabry NA, Omar EE. Corticosteroids and ICU course of community acquired pneumonia in Egyptian settings. Pharmacol Pharm. 2011;2:73–81. [Google Scholar]
- 25.Remmelts HH, Meijvis SC, Heijligenberg R, Rijkers GT, Oosterheert JJ, Bos WJ, et al. Biomarkers define the clinical response to dexamethasone in community-acquired pneumonia. J Infect. 2012;65(1):25–31. 10.1016/j.jinf.2012.03.008 [DOI] [PubMed] [Google Scholar]
- 26.Nafae RM, Ragab MI, Amany FM, Rashed SB. Adjuvant role of corticosteroids in the treatment of community-acquired pneumonia. Egyptian Journal of Chest Diseases and Tuberculosis. 2013;62:439–45. [Google Scholar]
- 27.Blum CA, Nigro N, Briel M, Schuetz P, Ullmer E, Suter-Widmer I, et al. Adjunct prednisone therapy for patients with community-acquired pneumonia: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2015;385(9977):1511–8. 10.1016/S0140-6736(14)62447-8 [DOI] [PubMed] [Google Scholar]
- 28.Torres A, Sibila O, Ferrer M, Polverino E, Menendez R, Mensa J, et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: a randomized clinical trial. Jama. 2015;313(7):677–86. 10.1001/jama.2015.88 [DOI] [PubMed] [Google Scholar]
- 29.Siempos II, Vardakas KZ, Kopterides P, Falagas ME. Adjunctive therapies for community-acquired pneumonia: a systematic review. J Antimicrob Chemother. 2008;62(4):661–8. 10.1093/jac/dkn283 [DOI] [PubMed] [Google Scholar]
- 30.Salluh JI, Shinotsuka CR, Soares M, Bozza FA, Lapa e Silva JR, Tura BR, et al. Cortisol levels and adrenal response in severe community-acquired pneumonia: a systematic review of the literature. J Crit Care. 2010;25(3):541, e1–8. [DOI] [PubMed] [Google Scholar]
- 31.Chen Y, Li K, Pu H, Wu T. Corticosteroids for pneumonia. Cochrane Database Syst Rev. 2011(3):Cd007720 10.1002/14651858.CD007720.pub2 [DOI] [PubMed] [Google Scholar]
- 32.Nie W, Zhang Y, Cheng J, Xiu Q. Corticosteroids in the treatment of community-acquired pneumonia in adults: a meta-analysis. PLoS One. 2012;7(10):e47926 10.1371/journal.pone.0047926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shafiq M, Mansoor MS, Khan AA, Sohail MR, Murad MH. Adjuvant steroid therapy in community-acquired pneumonia: a systematic review and meta-analysis. J Hosp Med. 2013;8(2):68–75. 10.1002/jhm.1992 [DOI] [PubMed] [Google Scholar]
- 34.Gusmao-Flores D. Corticosteroid therapy for severe community-acquired pneumonia: a meta-analysis. Respir Care. 2014;59(8):e118. [DOI] [PubMed] [Google Scholar]
- 35.Horita N, Otsuka T, Haranaga S, Namkoong H, Miki M, Miyashita N, et al. Adjunctive Systemic Corticosteroids for Hospitalized Community-Acquired Pneumonia: Systematic Review and Meta-Analysis 2015 Update. Sci Rep. 2015;5:14061 10.1038/srep14061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siemieniuk RA, Meade MO, Alonso-Coello P, Briel M, Evaniew N, Prasad M, et al. Corticosteroid Therapy for Patients Hospitalized With Community-Acquired Pneumonia: A Systematic Review and Meta-analysis. Ann Intern Med. 2015;163(7):519–28. 10.7326/M15-0715 [DOI] [PubMed] [Google Scholar]
- 37.Wan YD, Sun TW, Liu ZQ, Zhang SG, Wang LX, Kan QC. Efficacy and Safety of Corticosteroids for Community-Acquired Pneumonia: A Systematic Review and Meta-Analysis. Chest. 2016;149(1):209–19. 10.1378/chest.15-1733 [DOI] [PubMed] [Google Scholar]
- 38.Lim WS, van der Eerden MM, Laing R, Boersma WG, Karalus N, Town GI, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58(5):377–82. 10.1136/thorax.58.5.377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fine MJ, Auble TE, Yealy DM, Hanusa BH, Weissfeld LA, Singer DE, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336(4):243–50. 10.1056/NEJM199701233360402 [DOI] [PubMed] [Google Scholar]
- 40.Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44 Suppl 2:S27–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.BTS Guidelines for the Management of Community Acquired Pneumonia in Adults. Thorax. 2001;56 Suppl 4:Iv1–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marti C, Garin N, Grosgurin O, Poncet A, Combescure C, Carballo S, et al. Prediction of severe community-acquired pneumonia: a systematic review and meta-analysis. Crit Care. 2012;16(4):R141 10.1186/cc11447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Charles PG, Wolfe R, Whitby M, Fine MJ, Fuller AJ, Stirling R, et al. SMART-COP: a tool for predicting the need for intensive respiratory or vasopressor support in community-acquired pneumonia. Clin Infect Dis. 2008;47(3):375–84. 10.1086/589754 [DOI] [PubMed] [Google Scholar]
- 44.Rello J, Rodriguez A, Lisboa T, Gallego M, Lujan M, Wunderink R. PIRO score for community-acquired pneumonia: a new prediction rule for assessment of severity in intensive care unit patients with community-acquired pneumonia. Crit Care Med. 2009;37(2):456–62. 10.1097/CCM.0b013e318194b021 [DOI] [PubMed] [Google Scholar]
- 45.Sligl WI, Marrie TJ. Severe community-acquired pneumonia. Crit Care Clin. 2013;29(3):563–601. 10.1016/j.ccc.2013.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13 10.1186/1471-2288-5-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj. 2003;327(7414):557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sterne JA, Egger M, Smith GD. Systematic reviews in health care: Investigating and dealing with publication and other biases in meta-analysis. Bmj. 2001;323(7304):101–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. Bmj. 2011;343:d5928 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fernandez-Botran R, Uriarte SM, Arnold FW, Rodriguez-Hernandez L, Rane MJ, Peyrani P, et al. Contrasting inflammatory responses in severe and non-severe community-acquired pneumonia. Inflammation. 2014;37(4):1158–66. 10.1007/s10753-014-9840-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Galon J, Franchimont D, Hiroi N, Frey G, Boettner A, Ehrhart-Bornstein M, et al. Gene profiling reveals unknown enhancing and suppressive actions of glucocorticoids on immune cells. Faseb j. 2002;16(1):61–71. 10.1096/fj.01-0245com [DOI] [PubMed] [Google Scholar]
- 52.Sibila O, Luna CM, Agusti C, Baquero S, Gando S, Patron JR, et al. Effects of glucocorticoids in ventilated piglets with severe pneumonia. The European respiratory journal. 2008;32(4):1037–46. 10.1183/09031936.00009208 [DOI] [PubMed] [Google Scholar]
- 53.Lamontagne F, Briel M, Guyatt GH, Cook DJ, Bhatnagar N, Meade M. Corticosteroid therapy for acute lung injury, acute respiratory distress syndrome, and severe pneumonia: a meta-analysis of randomized controlled trials. J Crit Care. 2010;25(3):420–35. 10.1016/j.jcrc.2009.08.009 [DOI] [PubMed] [Google Scholar]
- 54.Carratala J, Garcia-Vidal C, Ortega L, Fernandez-Sabe N, Clemente M, Albero G, et al. Effect of a 3-step critical pathway to reduce duration of intravenous antibiotic therapy and length of stay in community-acquired pneumonia: a randomized controlled trial. Arch Intern Med. 2012;172(12):922–8. 10.1001/archinternmed.2012.1690 [DOI] [PubMed] [Google Scholar]
- 55.Annane D, Bellissant E, Bollaert PE, Briegel J, Confalonieri M, De Gaudio R, et al. Corticosteroids in the treatment of severe sepsis and septic shock in adults: a systematic review. Jama. 2009;301(22):2362–75. 10.1001/jama.2009.815 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Informantion files.