Abstract
Although emotional intensity powerfully challenges regulatory strategies, its influence remains largely unexplored in affective-neuroscience. Accordingly, the present study addressed the moderating role of emotional intensity in two regulatory stages—implementation (during regulation) and pre-implementation (prior to regulation), of two major cognitive regulatory strategies—distraction and reappraisal. According to our framework, because distraction implementation involves early attentional disengagement from emotional information before it gathers force, in high-intensity it should be more effective in the short-term, relative to reappraisal, which modulates emotional processing only at a late semantic meaning phase. Supporting findings showed that in high (but not low) intensity, distraction implementation resulted in stronger modulation of negative experience, reduced neural emotional processing (centro-parietal late positive potential, LPP), with suggestive evidence for less cognitive effort (frontal-LPP), relative to reappraisal. Related pre-implementation findings confirmed that anticipating regulation of high-intensity stimuli resulted in distraction (over reappraisal) preference. In contrast, anticipating regulation of low-intensity stimuli resulted in reappraisal (over distraction) preference, which is most beneficial for long-term adaptation. Furthermore, anticipating cognitively demanding regulation, either in cases of regulating counter to these preferences or via the more effortful strategy of reappraisal, enhanced neural attentional resource allocation (Stimulus Preceding Negativity). Broad implications are discussed.
Keywords: emotion regulation, EEG, attentional distraction, cognitive reappraisal, emotional intensity
INTRODUCTION
Imagine a man with aerophobia who is regulating his dread during a flight, by trying to think about his plans when he gets back home, thereby distracting from the situation. Alternatively, he might reappraise the situation by reminding himself that flights have become very safe these days. Even before the flight, while waiting for boarding, he had probably anticipated regulating his fear once he gets on the plane. In thinking about this man and his regulatory efforts, we were silent about a central contextual element, namely the emotional intensity he is facing. Specifically, our view is likely to be significantly different if we imagine him regulating or anticipating regulation of a flight that would involve emergency landing, relative to a mildly challenging landing.
Given the powerful impact emotional intensity should have on different emotion regulatory processes, it is surprising that the field of affective neuroscience has largely disregarded it. Accordingly, the main goal of the present study was to provide and test a comprehensive account of the moderating role of emotional intensity in two central regulatory stages—implementation (during active regulation) and pre-implementation (prior to active regulation), among two major cognitive emotion regulation strategies (attentional distraction and cognitive reappraisal).
In what follows, we present a recent conceptual framework and preliminary indirect behavioral evidence for the moderating role of emotional intensity in implementation and pre-implementation stages of distraction and reappraisal (Sheppes and Gross, 2011, 2012; Sheppes and Levin, 2013). We then review relevant neuroscience studies that did not evaluate emotional intensity. The present study provides the first direct test of the electro-cortical correlates of implementation and pre-implementation processes of distraction and reappraisal, as a function of differing emotional intensities.
THE ROLE OF EMOTIONAL INTENSITY IN IMPLEMENTATION REGULATORY PROCESSES
Our recently developed framework, that extends the process model of emotion regulation (Gross, 2014), provides conceptual logic and preliminary indirect behavioral evidence for the moderating role of emotional intensity in the implementation of distraction and reappraisal (Sheppes and Gross, 2011, 2012). Implementation, which is the most studied stage in emotion regulation, refers to the actual execution and consequences of different cognitive regulatory strategies (Hajcak et al., 2010; Ochsner et al., 2012; Buhle et al., 2014 for reviews). According to our framework, the basic differential potency of high vs low emotional intensity information, as well as the amount of elaborated processing it underwent across time prior to competing with regulatory strategies, determine regulatory efficacy. Distraction, which competes with emotional information at an early attentional selection stage, involves disengagement from emotional content before it gathers substantial force, by producing unrelated neutral thoughts. Alternatively, in Reappraisal emotional information is attended prior to a late competition at a semantic meaning phase, where less negative interpretations are formed. Consequently, in high-intensity situations, it is assumed that early attentional selection competition between distraction and basic potent emotional information that gathered minimal force prior to being distracted, is likely to result in more successful short-term emotional modulation, relative to a late semantic meaning competition between reappraisal and basic potent information that also gained additional force prior to being reappraised. However, in low-intensity situations, late competition between less potent emotional information and reappraisal is also likely to be successful.
Behavioral support for our framework comes from several studies, which have shown that in high emotional intensity, distraction resulted in stronger short-term modulation of self-reported negative experience compared with reappraisal, whereas in low emotional intensity both strategies resulted in equal modulation of negative experience (Sheppes and Meiran, 2007; Sheppes et al., 2014b). Although behavioral self-report ratings are important outcome measures, they do not provide information about underlying processes.
Given that in implementation processes emotional information (that varies in basic potency) undergoes differential processing across time prior to regulation, event related potentials (ERPs), which map cognitive-affective processes with high temporal resolution, have provided valuable insights (Hajcak et al., 2012 for a review). Particularly the centro-parietal late positive potential (LPP) component, that evolves from ∼300 ms and persists for several seconds following stimulus onset, has been shown to reflect enhanced processing of emotionally arousing compared with neutral stimuli (Hajcak et al., 2010 for a review). In the context of emotion regulation implementation, the typical finding of modulated centro-parietal LPP during regulation relative to a no-regulation condition was interpreted as indexing regulatory success (Hajcak and Nieuwenhuis, 2006; Moser et al., 2006; Dennis and Hajcak, 2009). Importantly, several recent studies showed that distraction strongly modulated the centro-parietal LPP from its very beginning, whereas reappraisal only modulated its late phase (Thiruchselvam et al., 2011; Paul et al., 2013; Schönfelder et al., 2014). Critically, while these prior ERP studies provide evidence for differential emotional modulation of distraction and reappraisal (McRae et al., 2010; Kanske et al., 2011 for congruent neuroimaging findings), none of these studies evaluated the important moderating role of emotional intensity. To that end, in this study, we examined whether the enhanced early centro-parietal LPP modulation in distraction relative to reappraisal should emerge particularly in high (but not low) emotional intensity.
In addition to showing differential emotional modulation, regulatory strategies require differential cognitive effort to operate. Our framework predicts that the late semantic meaning competition between reappraisal and emotional information would generally require increased cognitive effort, relative to the early competition in distraction, and that this increased effort would evince particularly in high-intensity situations. Supporting behavioral findings showed that in high-intensity, relative to distraction, reappraisal implementation resulted in increased self-reported effort and resource depletion (Sheppes and Meiran, 2008; Sheppes et al., 2014b). In two recent studies that did not manipulate emotional intensity, preliminary neural evidence for increased cognitive effort associated with reappraisal implementation was demonstrated by an early enhancement in LPP magnitudes particularly at frontal sites (i.e. frontal-LPP, Bernat et al., 2011; Moser et al., 2014). Given that empirical evidence with the frontal-LPP is still limited, in this study, we tentatively explored whether increased frontal-LPP magnitudes would evince for reappraisal but not distraction and particularly when reappraising high-intensity stimuli.
THE ROLE OF EMOTIONAL INTENSITY IN PRE-IMPLEMENTATION REGULATORY PROCESSES
Pre-implementation, which has been only infrequently studied, refers to processes which precede the actual execution of emotion regulation strategies (e.g. Denny et al., 2014). Our framework was recently extended to provide conceptual logic and preliminary indirect behavioral evidence for the moderating role of emotional intensity in one pre-implementation process, namely regulatory preferences (Sheppes and Levin, 2013 for review). Regulatory preferences for distraction and reappraisal are driven by anticipating the consequences of implementing these strategies in different contexts of high and low emotional intensities (Sheppes and Levin, 2013). Specifically, two behavioral studies (Sheppes et al., 2011, 2014a) showed that when anticipating upcoming regulation of high-intensity situations distraction was preferred over reappraisal because it results in enhanced short-term emotional modulation. In contrast, although in low-intensity both strategies are expected to be equally efficient in the short-term, reappraisal was preferred over distraction because the engagement with emotional information in reappraisal (but not in distraction), which allows to make sense of emotional events, is important for long-term adaptation (Wilson and Gilbert, 2008; MacNamara et al., 2011; Thiruchselvam et al., 2011; Blechert et al., 2012).
In this study, we explored the electro-cortical activity associated with pre-implementory anticipation for regulating via distraction and reappraisal in high vs low emotional intensities (i.e. regulatory preferences). Prior studies found that relative to pre-implementory anticipation to passively watch emotional stimuli, anticipating to regulate emotions enhanced the Stimulus Preceding Negativity (SPN, Moser et al., 2009; Thiruchselvam et al., 2011; Brunia et al., 2012 for a review). The SPN has been interpreted as reflecting enhanced orienting and recruitment of attentional resources (Lin et al., 2014) prior to the regulatory task, possibly because regulation is anticipated to be more cognitively demanding, relative to not regulating. Critically, while these studies provided anticipatory information regarding regulatory strategies, information about emotional intensity was not provided.
Providing anticipatory information concerning emotional intensity in addition to information regarding regulatory strategies may form regulatory conditions that are expected to be more cognitively demanding, and thus to induce enhanced orienting and attentional allocation during anticipation (greater SPNs). Specifically, we suggest that relative to anticipating to regulate in accordance with regulatory preferences (i.e. anticipating to reappraise low-intensity stimuli or distract high-intensity stimuli), anticipating to regulate counter to regulatory preferences (i.e. anticipating to reappraise high-intensity stimuli or distract low-intensity stimuli) may be more cognitively demanding. Empirical support for this argument comes from finding increased Contingent Negative Variation (an anticipatory ERP component that conceptually overlaps with the SPN) magnitudes when anticipating (prior to execution) increased cognitive demand in an incongruent Stroop condition [i.e. color naming that required overriding a default word reading response (Hart et al., 2012)].
THE PRESENT STUDY
The main goal of this study was to examine theoretically derived predictions regarding the moderating role of emotional intensity in implementation and pre-implementation processes of distraction and reappraisal. Within implementation, consistent with our previous behavioral findings (Sheppes and Meiran, 2007; Sheppes et al., 2014b), we expected that in high (but not low) intensity, distraction would result in less negative experience relative to reappraisal. Neurally, we expected that in high (but not low) intensity, distraction would result in modulation of the early phase of the centro-parietal LPP compared with reappraisal, which in turn was expected to modulate only the late phase of the centro-parietal LPP. Secondary hypothesis involved exploring whether increased frontal-LPP magnitudes would evince for reappraisal but not distraction, and particularly when reappraising high-intensity stimuli.
Within pre-implementation, consistent with our previous behavioral findings (Sheppes et al., 2011, 2014a), we expected that participants would prefer distraction over reappraisal when anticipating to regulate high-intensity pictures and would prefer reappraisal over distraction when anticipating to regulate low-intensity pictures. Neurally, we expected that relative to anticipating to regulate in accordance with regulatory preferences, demanding conditions of anticipating to override regulatory preferences would result in enhanced SPN magnitudes.
METHOD
Participants
Thirty healthy adults with normal or corrected-to-normal vision participated in the experiment. One participant had poor electroencephalography (EEG) data quality, which did not allow for artifact correction. Two participants were excluded due to excessive artifacts (>49% of rejected trials) or poor compliance with regulation instructions1. Therefore, the final sample consisted of 27 participants (12 men2).
Stimuli
One hundred eighty negative pictures were mostly chosen from previously validated pictorial datasets3 (IAPS: Lang et al., 2008; GAPED: Dan-Glauser and Scherer, 2011; EmoPicS: Wessa et al., 2010; and several additional pictures validated in our lab). High-intensity pictures (n = 90, Marousal = 6.51, Mvalence = 2.01) and Low-intensity pictures (n = 90, Marousal = 4.72, Mvalence = 3.38) were significantly different in valence and arousal normative ratings (both Fs > 423, ps < 0.001; c.f. Sheppes et al., 2011, 2014a). Picture content included sadness, disgust, threat, fear and mutilations and was matched for the high- and low-intensity categories whenever possible4.
Procedure
EEG task
Following initial EEG setup, participants learned (four trials) and practiced (six trials) how to implement the different instruction types (Sheppes et al., 2014a for complete details). To ensure understanding of the instructions, during these phases participants talked out loud throughout implementation.
Distraction instructions involved disengaging attention from emotional pictures by producing unrelated neutral thoughts (i.e. visualizing geometric shapes or daily activities). Reappraisal instructions involved engaging attention in emotional pictures but changing their emotional meaning (i.e. thinking that the situation would get better over time or focusing on an aspect of the situation that is less negative). In order to maintain the fundamental difference between disengagement distraction and engagement reappraisal, we did not allow participants to form reality challenge reappraisals (e.g. construe an emotional situation as ‘fake’), which involve disengagement (Sheppes et al., 2014a, Study 6 for details). ‘Watch’ instructions involved allowing natural thoughts and feelings to arise while looking at the pictures.
The task consisted of 180 trials (six equally long blocks, separated by short breaks) that were equally divided into six experimental conditions (30 trials per condition) according to two independent variables: Emotional Intensity (High, Low) and Instruction Type (Distraction, Reappraisal, Watch). Pictures were randomly assigned to Instruction types, with no more than two consecutive trials of the same emotional intensity.
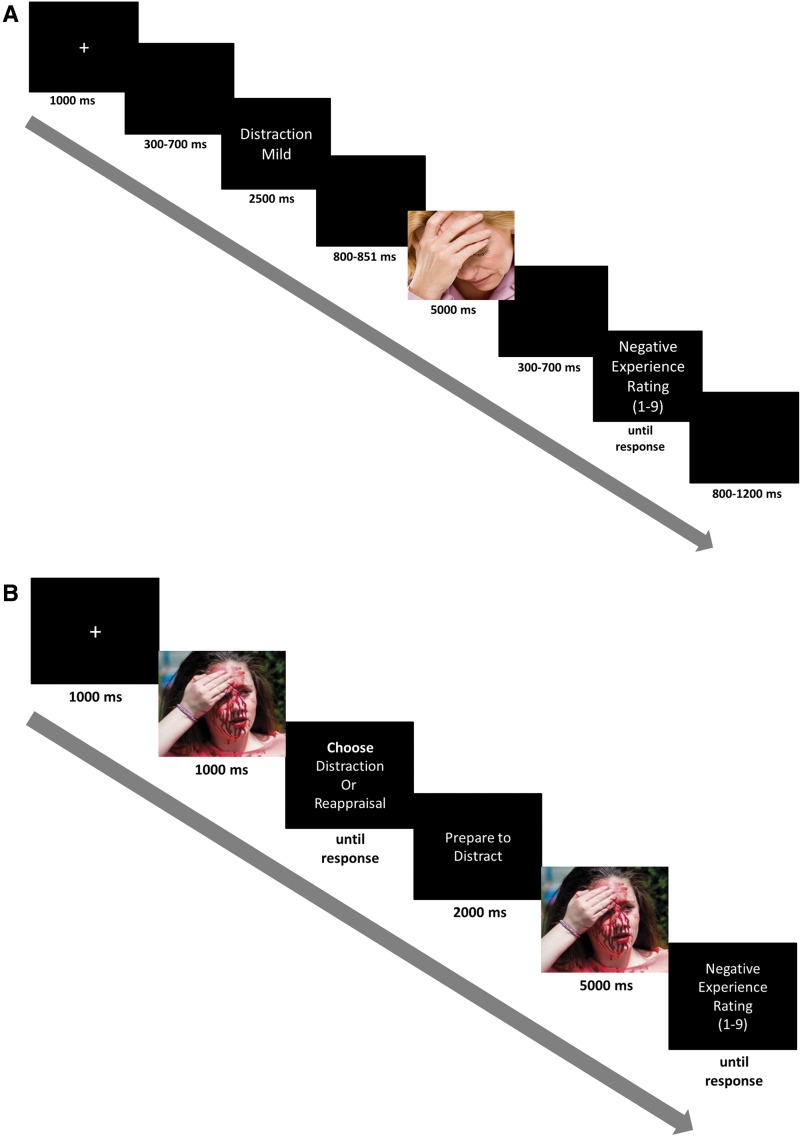
Trial sequence (Figure 1a for complete details) included a fixation cross, followed by a cue screen containing information about the upcoming intensity of the picture (‘Intense’ or ‘Mild’) and the required instruction type (‘Distraction’ or ‘Reappraisal’ or ‘Watch’), followed by picture presentation during which participants implemented the experimental instruction, followed by a rating screen in which participants rated their level of negative experience on a 1–9 scale (1 = ‘not negative at all’, 9 = ‘extremely negative’).
Fig. 1.
Trial structures. (A) Trial structure of the EEG task (an example of a low emotional intensity distraction trial). (B) Trial structure of the behavioral regulatory preference task (an example of a high emotional intensity distraction preference trial).
Behavioral regulatory preference task
After completing the EEG task, behavioral regulatory preferences were assessed by having participants freely choose between implementing distraction or reappraisal when anticipating high (15 pictures) and low (15 pictures) emotional pictures3 (Sheppes et al., 2011 for details). Trial sequence (Figure 1b for details) included a fixation cross, followed by a brief preview of an emotional picture, followed by a distraction vs. reappraisal choice preference screen, followed by picture presentation where participants implemented their chosen strategy.
Electrophysiological recordings, data reduction and analysis
EEG was recorded using a Biosemi ActiveTwo recording system (Biosemi B. V., Amsterdam, The Netherlands). Sixty-four scalp-electrodes at locations of the extended 10–20 system were used, as well as one electrode on each of the left and right mastoids. The horizontal electrooculogram (EOG) was recorded from electrodes placed ∼1 cm to the left and right of the external canthi, and the vertical EOG was recorded from an electrode placed beneath the left eye. The voltage from each electrode site was referenced online with respect to the Common Mode Sense/ Driven Right Leg (CMS/DRL) electrodes. EEG data were sampled at 256 Hz.
Offline signal processing was performed using EEGLAB and ERPLAB Toolbox (Delorme and Makeig, 2004; Lopez-Calderon and Luck, 2014). All electrodes were re-referenced to the average activity of the left and right mastoids. Continuous EEG data were band-pass filtered (cutoffs: 0.05–20Hz; 12 dB/oct rolloff). EEG recordings were corrected for eye-movement artifacts using an independent component analysis approach (Delorme and Makeig, 2004; Mennes et al., 2010).
For the SPN analysis, the mean of a 200 ms pre-cue baseline was subtracted from the 2500 ms post-cue waveform. Similarly, for the centro-parietal and frontal-LPP a 200 ms pre-image baseline was subtracted from the 5000 ms image waveform. Trials containing activity exceeding 80 µV within 200 ms were excluded. The mean rejection rate was 4.08%, SE = 0.07 for the SPN analysis and 3.12%, SE = 0.06 for the centro-parietal and frontal-LPP analysis and did not vary as a function of condition [all F’s < 1].
The centro-parietal LPP was measured as the average activity of Pz and CPz, the two electrodes most consistently reported for this component (Hajcak et al., 2010 for a review). Following prior studies showing differential temporal modulation for distraction and reappraisal (Thiruchselvam et al., 2011; Paul et. al., 2013), we divided the centro-parietal LPP period into early and late time windows. The early window was defined as the average activity between 300 and 1700 ms post-picture onset, similar to the time window used by Paul et al. (2013). The late window began at 1700 ms and was of the same length as the early window. Our decision on these windows is also congruent with Thiruchselvam et al. (2011), who found an early centro-parietal LPP modulation for distraction at 300–500 ms (which closely matches the beginning of our early window), and late centro-parietal LPP modulation for reappraisal at 1500–1700 ms (which closely matches the beginning of our late window). Following Moser et al. (2014), the frontal-LPP was measured at Fz electrode between 800 and 1100 ms following picture onset.
Consistent with prior studies which have defined the fronto-parietal midline electrode sites as most relevant for examining SPN effects, we measured the SPN at Fz, FCz, Cz, CPz and Pz electrode cites. Because the scalp topography of the SPN diverges to some extent as a function of task demands (Boxtel and Böcker, 2004), within these electrode sites some variation in maximal topography has evinced with some studies finding maximal SPN amplitudes at Fz or FCz electrode sites (Moser et al., 2009; Thiruchselvam et al., 2011; but see Moser et al., 2014 who focused on centro-frontal electrodes and did not find an SPN effect), and other studies finding the SPN at frontal and/or parietal electrode sites (Poli et al., 2007; Buodo et al., 2012; Seidel et al., 2015). Consistent with some of these prior studies, in our study the SPN was quantified at Pz, where it was also maximal.
We measured the early phase of the SPN because it represents processing of the indicative anticipatory cue (Boxtel and Böcker, 2004; Moser et al., 2009, 2014) and precludes actual strategy preparation, which enables a clear conceptual differentiation between pre-implementation and implementation stages. In the most general and systematic review on the SPN (Boxtel and Böcker, 2004), as well as in specific studies on anticipation for emotion regulation (Moser et al., 2009, 2014), the time window that has been considered to represent the early SPN was around 1000 ms post-cue onset. Accordingly and also congruent with visual inspection, the 900–1300 ms time window was subjected to analysis.
RESULTS
Implementation processes
Behavioral measure of negative experience
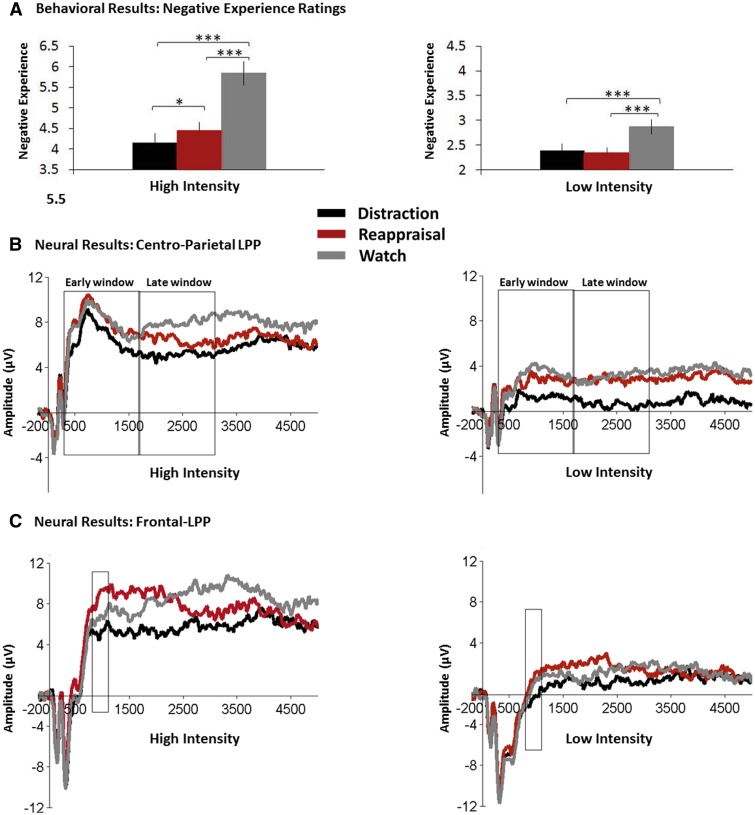
First, we confirmed our emotional intensity categorization in finding that high-intensity pictures (M = 4.82, SE = 0.37) were experienced as more negative than low-intensity pictures (M = 2.53, SE = 0.21) [F(1,26) = 236.99, P < 0.0000001, = 0.90]. To test the efficacy of distraction and reappraisal in high and low emotional intensities, as reflected in behavioral negative experience ratings, we employed a 2 × 3 analysis of variance (ANOVA) with Emotional-Intensity (high, low) and Instruction-Type (distraction, reappraisal, watch) as repeated measures factors. Supporting our conceptual framework and previous findings (Sheppes and Meiran, 2007; Sheppes et al., 2014b), we found a significant Emotional-Intensity × Instruction-Type interaction [F(2,52) = 19.16, P < 0.00001, = 0.42; Figure 2a]. As expected, follow-up comparisons showed that in high-intensity, although distraction (M = 4.16, SE = 0.22) [F(1,26) = 57.13, P < 0.0000001, = 0.69] and reappraisal (M = 4.45, SE = 0.2) [F(1,26) = 41.53, P < 0.00001, = 0.61] were both efficient in reducing negative experience compared with watch (M = 5.84, SE = 0.29), distraction’s efficacy relative to watch was greater than that of reappraisal vs watch [F(1,26) = 7.51, P < 0.02, = 0.22]. Confirming our prediction for low-intensity, we found that both strategies were efficient in reducing negative experience, relative to watch (M = 2.87, SE = 0.15): distraction (M = 2.38, SE = 0.14) [F(1,26) = 20, P < 0.001, = 0.43]; reappraisal (M = 2.34, SE = 0.11) [F(1,26) = 22.47, P < 0.0001, = 0.46], with no difference between the two regulatory options [F(1,26) < 1].
Fig. 2.
Implementation findings. (A) Negative experience ratings for Distraction, Reappraisal and Watch in high and low emotional intensities. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Error bars represent standard errors. (B) Picture-locked neural early and late window LPP amplitudes for Distraction, Reappraisal and Watch in high and low emotional intensities. Waveforms are averaged across Pz and CPz electrodes. The rectangles indicate the time window submitted to statistical analysis (i.e. 300–1700 ms for the early time window and1700–3100 ms for the late time window). The x-axis runs from the beginning of the baseline (−200 ms pre picture onset) to the end of the picture presentation (5000 ms). (C) Picture-locked frontal-LPP amplitudes at Fz electrode for Distraction, Reappraisal and Watch in high and low emotional intensities. The rectangles indicate the time window submitted to statistical analysis (i.e. 800–1100 ms). The x-axis runs from the beginning of the baseline (−200 ms pre picture onset) to the end of the picture presentation (5000 ms).
Neural measures of regulatory modulation and effort: centro-parietal and frontal-LPP analysis
Prior to conducting the main analyses, we confirmed our emotional intensity categorization in finding that high-intensity pictures (M = 6.87, SE = 0.95) produced greater centro-parietal LPPs than low-intensity pictures (M = 2.1, SE = 0.94) [F(1,26) = 120.82, P < 0.0000001, = 0.82]. To test whether the neural modulation differences between distraction and reappraisal differ in high and low emotional intensities, we employed a 2 × 2 × 3 ANOVA with Time-Window (early, late), Emotional-Intensity (high, low) and Instruction-Type (distraction, reappraisal, watch) as repeated-measures factors. Congruent with our conceptual framework (Sheppes and Gross, 2011), we found a significant Time-Window × Emotional-Intensity × Instruction-Type interaction [F(2,52) = 4.46, P < 0.02, = 0.15; Figure 2b]. Decomposition of the significant three-way interaction followed our prediction that in high [F(2,52) = 5.71, P < 0.01, = 0.18] but not in low-intensity [F(2,52) = 1.37, n.s.], distraction and reappraisal would differ in the time window in which they have their primary impact on the centro-parietal LPP. Follow-up comparisons tested our hypothesis that in high-intensity, distraction would modulate the centro-parietal LPP earlier than reappraisal. As predicted, relative to watch (early window: M = 7.63, SE = 0.85, late window: M = 7.91, SE = 0.97), distraction successfully modulated the centro-paretal LPP at both early (M = 6.36, SE = 1.02) [F(1,26) = 4.29, P < 0.05, = 0.14] and late (M = 5.1, SE = 0.96) [F(1,26) = 9.34, P < 0.01, = 0.26] time windows. In contrast, relative to watch, reappraisal did not modulate the centro-parietal LPP during the early time window (M = 7.85, SE = 0.92) [F(1,26) < 1], but successfully modulated the centro-parietal LPP during the late time window (M = 6.38, SE = 1.01) [F(1,26) = 5.48, P < 0.03, = 0.17]. Last, although in low-intensity we did not expect and did not find a simple Time-Window × Instruction-Type interaction [F(2,52) = 1.37, n.s.], the trend in means suggested that while distraction successfully modulated the centro-parietal LPP relative to watch, unexpectedly reappraisal did not.
Secondary analyses that estimate differential requirement for cognitive effort were carried out. Replicating Moser et al. (2014) and congruent with our prediction that reappraisal would be generally effortful, we found a significant main effect of Instruction-Type [F(2,52) = 5.97, P < 0.005, = 0.19], where relative to watch (M = 3.12, SE = 1.59) there was a marginally significant difference in frontal-LPP magnitudes for reappraisal (M = 4.17, SE = 1.39) [F(1,26) = 3.88, P = 0.058] but not distraction (M = 2.1, SE = 1.74) [F(1,26) = 2.73, n.s.], that produced the lowest frontal-LPP magnitudes. In addition, given that we had a priori predictions that particularly in high-intensity, relative to watch reappraisal but not distraction would result in enhanced frontal-LPP amplitudes, we conducted planned comparisons although the Emotional-Intensity × Instruction-Type interaction was not significant [F(2,52) < 1]. Enhanced requirement for cognitive effort was evident in finding higher frontal-LPP amplitudes during reappraisal in high-intensity (M = 7.1, SE = 1.16), compared with watch (M = 5.57, SE = 1.18) [F(1,26) = 4.33, P < 0.05, = 0.14]. Importantly, congruent with our hypothesis in high-intensity distraction (M = 4.58, SE = 1.42) was not associated with enhanced cognitive effort relative to watch [F(1,26) = 1.74, n.s.]. Notably, in low-intensity we did not expect and did not find enhanced frontal-LPP magnitudes for reappraisal (M = 1.24, SE = 0.98) or distraction (M = −0.39, SE = 1.12) relative to watch (M = 0.67, SE = 1.23) [both Fs < 1.7]. Frontal-LPP results are depicted in Figure 2c.
Pre-implementation processes
Behavioral measure of regulatory preferences
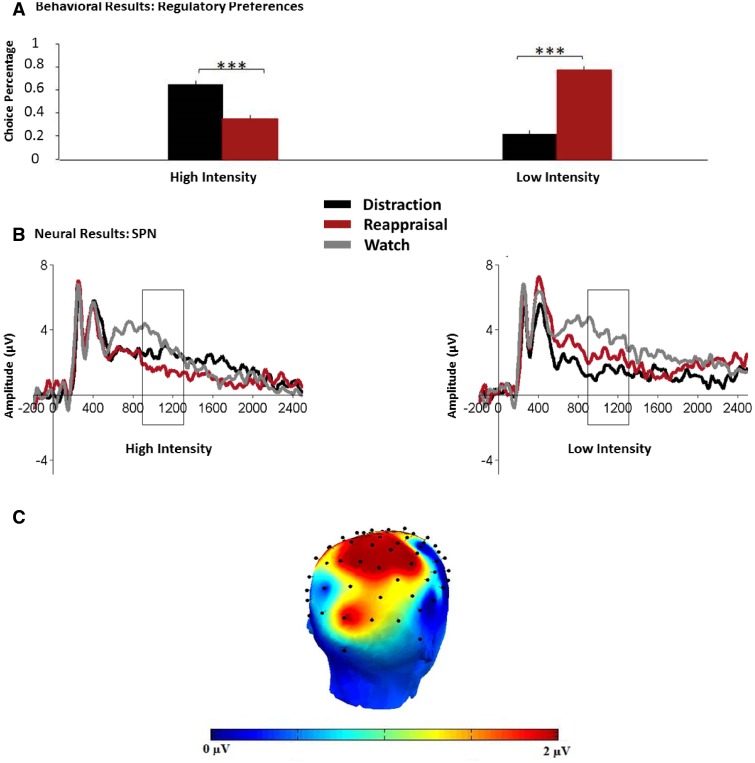
Consistent with our conceptual framework and previous findings (Sheppes et al., 2011, 2014a), we found that 89% (24/27) of participants preferred distraction (M = 64.9%) over reappraisal (M = 35.1%) when anticipating regulation of high-intensity pictures [t(26) = 4.94, P < 0.0001, each different from M = 50%, d = 0.97], but 96% (26/27) of participants preferred reappraisal (M = 77.9%) over distraction (M = 22.1%) when anticipating regulation of low-intensity pictures [t(26) = 9.47, P < 0.0000001, d = 1.86; Figure 3a].
Fig. 3.
Pre-implementation findings. (A) Percentage of trials participants behaviorally chose distraction or reappraisal in high and low emotional intensities. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Error bars represent standard errors. (B) SPN amplitudes during instruction cues at Pz in high and low emotional intensities. The rectangles indicate the time window submitted to statistical analysis (i.e. 900–1300 ms). The x-axis runs from the beginning of the baseline (−200 ms pre-cue onset) to the end of the cue presentation (2500 ms). (C) Head map of the SPN topographical distribution. Voltage difference score for the interaction between Emotional Intensity and Instruction type was calculated as: (distraction high minus reappraisal high)—(distraction low minus reappraisal low).
Neural measure of anticipatory processes: SPN analysis
To test whether the SPN is influenced by the anticipation for regulating emotions in accordance with or counter to regulatory preferences, we employed a 2 × 3 repeated-measures ANOVA with Emotional-Intensity (high, low) and Instruction-Type (distraction, reappraisal, watch) as factors. Confirming our prediction, we found a significant Emotional-Intensity × Instruction-Type cross-over interaction [F(2,52) = 3.28, P < 0.05, = 0.11; Figure 3b]. Follow-up analyses showed that in high-intensity, relative to watch (M = 3.18, SE = 0.44) reappraisal (the non-preferred strategy, M = 1.55, SE = 0.54) resulted in larger SPNs [F(1,26) = 8.98, P < 0.01, = 0.26], whereas distraction (M = 2.57, SE = 0.59) did not [F(1,26) = 2.1, n.s.]. A mirroring pattern emerged in low-intensity, where relative to watch (M = 3.66, SE = 0.71), distraction (the non-preferred strategy, M = 1.47, SE = 0.59) resulted in larger SPNs [F(1,26) = 13.36, P< 0.01, = 0.34]. However, unexpectedly reappraisal (the preferred strategy in low-intensity, M = 2.15, SE = 0.61), also produced greater SPNs relative to watch [F(1,26) = 5.73, P < 0.03, = 0.18]. The topography of the SPN suggested that the effect we observed was most evident in parietal electrodes (Figure 3c), was also obtained at the centro-parietal CPz electrode site, with a diminishing trend at more frontal sites (Supplementary Figure S4).
DISCUSSION
To account for the largely neglected influence of emotional intensity in the study of emotion regulation, the present study provided the first direct test of our conceptual framework by illuminating the behavioral and electro-cortical correlates of implementation and pre-implementation processes of distraction and reappraisal when facing differing emotional intensities.
Regarding implementation processes, we replicated prior behavioral findings (Sheppes and Meiran, 2007; Sheppes et al., 2014b) in showing that in high (but not low) emotional intensity, when compared with watch, the early attentional competition between distraction and basic potent emotional information, resulted in enhanced short-term modulation of negative experience, relative to the late sematic meaning competition between reappraisal and potent emotional information. Importantly, this result was congruent with the novel finding of strong neural modulation of the early phase of the centro-parietal LPP for distraction relative to reappraisal (both compared with watch) in high-intensity. Moreover, there was suggestive evidence that compared with watch, reappraisal but not distraction implementation generally requires increased cognitive effort, and particularly in high-intensity, as reflected by enhanced frontal-LPP magnitudes.
Regarding pre-implementation processes, we replicated prior behavioral findings (Sheppes et al., 2011, 2014a) in showing that when anticipating regulation of high-intensity stimuli, distraction is preferred over reappraisal. However, when anticipating regulation of low-intensity stimuli, reappraisal is preferred over distraction. Importantly, our studies provided novel neural manifestation of pre-implementory anticipation for regulating in accordance with or counter to regulatory preferences. Congruent with our expectations, we observed a pattern of a cross-over interaction where in high-intensity, relative to watch, reappraisal (the non-preferred strategy) but not distraction resulted in enhanced SPNs, and in low-intensity, relative to watch distraction (the non-preferred strategy) but unexpectedly also reappraisal resulted in enhanced SPNs.
Our results showing a cross-over interaction, enhanced SPN magnitudes for reappraisal in high-intensity and for distraction in low-intensity (both relative to watch), best support our prediction that the requirement to override regulatory preferences is anticipated to be cognitively demanding. However, the unexpected finding that across both intensities (relative to watch) reappraisal resulted in enhanced SPN magnitudes may suggest that the requirement to implement reappraisal is also generally anticipated to be demanding (but see Thiruchselvam et al., 2011 who did not find SPN differences between distraction and reappraisal cues). Congruent with our results showing enhanced frontal-LPPs for reappraisal (but not distraction), it may be that anticipating the more effortful strategy of reappraisal generally required the recruitment of additional attentional resources during anticipation.
Congruent with several prior studies, our interpretation of the SPN findings focuses on its cognitive aspects (Moser et al., 2009; Lin et al., 2014). Specifically, we argued that the SPN reflects enhanced orienting and recruitment of attentional resources prior to cognitively demanding regulatory implementation. However, the SPN also has affective aspects (Hajcak et al., 2012) and may reflect enhanced anticipatory arousal, such as increased nervousness, prior to demanding regulatory implementation (Moser et al., 2014).
Understanding the moderating relationship between emotional intensity and regulatory forms during implementation is straightforward, because at this stage emotional stimuli are present and regulatory forms are active. However, in pre-implementation this relationship requires additional clarifications because at this stage emotional stimuli have not been presented and regulatory forms have not been activated. To bridge this gap, our account makes the assumption that anticipatory information regarding both the emotional intensity and the regulatory strategy is sufficient in order to induce emotional and cognitive responses that represent expecting the consequences of regulatory implementation in different intensities (Sheppes and Levin, 2013).
Empirical support for our assumption comes from prior studies showing that anticipating negative emotional stimuli (relative to less intense neutral stimuli), was sufficient in order to induce higher SPN magnitudes (Poli et al., 2007; Buodo et al., 2012) as well as strong reactions in emotional (i.e. insula and amygdala) brain regions (e.g. Onoda et al., 2008). Furthermore, anticipating the implementation of regulatory strategies was sufficient in order to induce higher SPN magnitudes (Thiruchselvam et al., 2011, Moser et al., 2009) as well as strong reactions in cognitive control (i.e. rostrolateral prefrontal cortex) brain regions (Denny et al., 2014). Furthermore, our prior behavioral studies showed that regulatory preferences represent individuals’ ability to anticipate the consequences of implementing regulatory strategies in different intensities (Sheppes et al., 2011, 2014a).
Our results have important clinical implications. Specifically, many forms of psychopathologies are characterized by high emotional intensity levels and impaired cognitive emotion regulation (Sheppes et al., 2015). Accordingly, our results add to several recent findings suggesting that particularly in highly stressful situations, engaging strategies such as reappraisal become less effective (Raio et al., 2013; Silvers et al., 2015). We extend these findings by showing that in these intense situations, effective disengaging strategies such as distraction can serve as a beneficial ‘first aid’ tool. However, since the engagement with emotional information in reappraisal allows facing emotional challenges in order to better deal with them in the future (Wilson and Gilbert, 2008), disengagement via distraction has critical shortcomings in promoting long-term mental health.
Despite the novel features of the study, it is important to mention several limitations. First, unexpectedly, in low-intensity, relative to watch reappraisal implementation did not modulate the centro-parietal LPP. It may be that for low-intensity the modulation of the centro-parietal LPP is not sensitive to reappraisal instructions. However, it is important to note that congruent with our hypotheses in low-intensity the interaction between instruction type and centro-parietal LPP time window was not significant, which suggests that any interpretation should be treated with caution.
Second, although we conceptually link between behavioral regulatory preferences and neural anticipatory SPN magnitudes, each was measured in different tasks and thus different cognitive processes are likely to be taking place. It is important to note, however, that conceptually, neural processing of regulatory cue instructions and behavioral regulatory preferences are pre-implementaory in the sense that they both involve anticipatory processes that precede actual regulatory implementation. Specifically, both processes involve anticipating in advance the consequences of implementing distraction and reappraisal when facing high vs low emotional intensities.
Third, it may be argued that during the anticipatory cue presentation, participants started already implementing distraction because only distraction implementation does not depend on the picture contents. However, we believe that focusing our SPN analysis on the early window maximizes our chances to preclude actual strategy preparation during anticipation (Boxtel and Böcker, 2004). Importantly, our actual results showing lower SPNs for distraction relative to reappraisal cues in high-intensity are incongruent with actual implementation that should have yielded higher SPNs for distraction (Moser et al., 2014).
Finally, in this study, participants were presented with only negative pictures, which poses habituation risk across time. However, the centro-parietal LPP mean amplitudes we observed were fairly similar in magnitude to those observed in previous studies that did include emotionally neutral stimuli (e.g. Thiruchselvam et al., 2011; Paul et al., 2013), which suggests that the presence of habituation effect in our study is likely to be minimal.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online
Conflicts of Interest
None declared.
Supplementary Material
Acknowledgments
This work was supported by the National Institute for Psychobiology in Israel, the Dylan Tauber Track (grant no. 242-13-14) awarded to G.S. We wish to thank the following research assistants for their help: Or Mandel, Shir Murciano, Amir Zahavi and Omer Shuval-Shakked.
Footnotes
1 Despite the reduced power due to increased noise, all of the results remained essentially unchanged when re-running all the main analyses including the excluded participants.
2 We re-ran all the analyses considering gender as an additional between-group variable and found no effects (all F’s < 1).
3 All picture codes are included in Supplementary materials.
4 Note that all analyses that decomposed interactions with intensity level involved comparing different instructions for each emotional intensity separately. Thus, possible content differences between high- and low-intensity pictures have no bearing on the results.
REFERENCES
- Bernat EM, Cadwallader M, Seo D, Vizueta N, Patrick CJ. Effects of instructed emotion regulation on valence, arousal, and attentional measures of affective processing. Developmental Neuropsychology. 2011;36(4):493–518. doi: 10.1080/87565641.2010.549881. [DOI] [PubMed] [Google Scholar]
- Blechert J, Sheppes G, Di Tella C, Williams H, Gross JJ. See what you think: reappraisal modulates behavioral and neural responses to social stimuli. Psychological Science. 2012;23(4):346–53. doi: 10.1177/0956797612438559. [DOI] [PubMed] [Google Scholar]
- van Boxtel GJ, Böcker KB. Cortical measures of anticipation. Journal of Psychophysiology. 2004;18:61–76. [Google Scholar]
- Brunia CH, van Boxtel GJ, Böcker KB. Negative slow waves as indices of anticipation: the bereitschaftspotential, the contingent negative variation, and the stimulus-preceding negativity. In: Luck SJ, Kappenman ES, editors. Oxford Handbook of ERP Components. New York: Oxford University Press; 2012. pp. 189–207. [Google Scholar]
- Buhle JT, Silvers JA, Wager TD, et al. Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cerebral Cortex. 2014;24:2981–90. doi: 10.1093/cercor/bht154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buodo G, Sarlo M, Poli S, et al. Emotional anticipation rather than processing is altered in patients with vasovagal syncope. Clinical Neurophysiology. 2012;123(7):1319–27. doi: 10.1016/j.clinph.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Dan-Glauser ES, Scherer KR. The Geneva affective picture database (GAPED): a new 730-picture database focusing on valence and normative significance. Behavior Research Methods. 2011;43(2):468–77. doi: 10.3758/s13428-011-0064-1. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods. 2004;134(1):9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Dennis TA, Hajcak G. The late positive potential: neurophysiological marker for emotion regulation in children. Journal of Child Psychology and Psychiatry. 2009;50(11):1373–83. doi: 10.1111/j.1469-7610.2009.02168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny BT, Ochsner KN, Weber J, Wager TD. Anticipatory brain activity predicts the success or failure of subsequent emotion regulation. Social Cognitive and Affective Neuroscience. 2014;9(4):403–11. doi: 10.1093/scan/nss148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ. Emotion regulation: conceptual and empirical foundation. In: Gross JJ, editor. Handbook of Emotion Regulation. 2nd edn. New York: Guilford; 2014. pp. 3–20. [Google Scholar]
- Hajcak G, MacNamara A, Olvet DM. Event-related potentials, emotion, and emotion regulation: an integrative review. Developmental Neuropsychology. 2010;35(2):129–55. doi: 10.1080/87565640903526504. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Nieuwenhuis S. Reappraisal modulates the electrocortical response to unpleasant pictures. Cognitive, Affective & Behavioral Neuroscience. 2006;6(4):291–7. doi: 10.3758/cabn.6.4.291. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Weinberg A, MacNamara A, Foti D. ERPs and the study of emotion. In: Luck SJ, Kappenman ES, editors. Oxford Handbook of ERP Components. New York: Oxford University Press; 2012. pp. 441–74. [Google Scholar]
- Hart SJ, Lucena N, Cleary KM, Belger A, Donkers FCL. Modulation of early and late event-related potentials by emotion. Frontiers in Integrative Neuroscience. 2012;6:102. doi: 10.3389/fnint.2012.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanske P, Heissler J, Schönfelder S, Bongers A, Wessa M. How to regulate emotion? Neural networks for reappraisal and distraction. Cerebral Cortex. 2011;21(6):1379–88. doi: 10.1093/cercor/bhq216. [DOI] [PubMed] [Google Scholar]
- Lang P, Bradley M, Cuthbert B. Technical report. A–8. Gainesville, FL: University of Florida; 2008. International affective picture system (IAPS): affective ratings of pictures and instruction manual. [Google Scholar]
- Lin H, Gao H, You J, et al. Larger N2 and smaller early contingent negative variation during the processing of uncertainty about future emotional events. International Journal of Psychophysiology. 2014;94(3):292–7. doi: 10.1016/j.ijpsycho.2014.10.004. [DOI] [PubMed] [Google Scholar]
- Lopez-Calderon J, Luck SJ. ERPLAB: an open-source toolbox for the analysis of event-related potentials. Frontiers in Human Neuroscience. 2014;8 doi: 10.3389/fnhum.2014.00213. 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNamara A, Ochsner KN, Hajcak G. Previously reappraised: the lasting effect of description type on picture-elicited electrocortical activity. Social Cognitive and Affective Neuroscience. 2011;6(3):348–58. doi: 10.1093/scan/nsq053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae K, Hughes B, Chopra S, Gabrieli JDE, Gross JJ, Ochsner KN. The neural bases of distraction and reappraisal. Journal of Cognitive Neuroscience. 2010;22(2):248–62. doi: 10.1162/jocn.2009.21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennes M, Wouters H, Vanrumste B, Lagae L, Stiers P. Validation of ICA as a tool to remove eye movement artifacts from EEG/ERP. Psychophysiology. 2010;47(6):1142–50. doi: 10.1111/j.1469-8986.2010.01015.x. [DOI] [PubMed] [Google Scholar]
- Moser JS, Hajcak G, Bukay E, Simons RF. Intentional modulation of emotional responding to unpleasant pictures: an ERP study. Psychophysiology. 2006;43(3):292–6. doi: 10.1111/j.1469-8986.2006.00402.x. [DOI] [PubMed] [Google Scholar]
- Moser JS, Hartwig R, Moran TP, Jendrusina AA, Kross E. Neural markers of positive reappraisal and their associations with trait reappraisal and worry. Journal of Abnormal Psychology. 2014;123(1):91–105. doi: 10.1037/a0035817. [DOI] [PubMed] [Google Scholar]
- Moser JS, Krompinger JW, Dietz J, Simons RF. Electrophysiological correlates of decreasing and increasing emotional responses to unpleasant pictures. Psychophysiology. 2009;46(1):17–27. doi: 10.1111/j.1469-8986.2008.00721.x. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences. 2012;1251:E1–24. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoda K, Okamoto Y, Toki S, et al. Anterior cingulate cortex modulates preparatory activation during certain anticipation of negative picture. Neuropsychologia. 2008;46(1):102–10. doi: 10.1016/j.neuropsychologia.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Paul S, Simon D, Kniesche R, Kathmann N, Endrass T. Timing effects of antecedent- and response-focused emotion regulation strategies. Biological Psychology. 2013;94(1):136–42. doi: 10.1016/j.biopsycho.2013.05.019. [DOI] [PubMed] [Google Scholar]
- Poli S, Sarlo M, Bortoletto M, Buodo G, Palomba D. Stimulus-preceding negativity and heart rate changes in anticipation of affective pictures. International Journal of Psychophysiology. 2007;65(1):32–9. doi: 10.1016/j.ijpsycho.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Raio CM, Orederu TA, Palazzolo L, Shurick AA, Phelps EA. Cognitive emotion regulation fails the stress test. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(37):15139–44. doi: 10.1073/pnas.1305706110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönfelder S, Kanske P, Heissler J, Wessa M. Time course of emotion-related responding during distraction and reappraisal. Social Cognitive and Affective Neuroscience. 2014;9(9):1310–19. doi: 10.1093/scan/nst116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel EM, Pfabigan DM, Hahn A, et al. Uncertainty during pain anticipation: the adaptive value of preparatory processes. Human Brain Mapping. 2015;36(2):744–55. doi: 10.1002/hbm.22661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppes G, Brady WJ, Samson AC. In (visual) search for a new distraction: the efficiency of a novel attentional deployment versus semantic meaning regulation strategies. Frontiers in Psychology. 2014b;5:346. doi: 10.3389/fpsyg.2014.00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppes G, Gross JJ. Is timing everything? Temporal considerations in emotion regulation. Personality and Social Psychology Review. 2011;15(4):319–31. doi: 10.1177/1088868310395778. [DOI] [PubMed] [Google Scholar]
- Sheppes G, Gross JJ. Emotion regulation effectiveness: what works when. In: Tennen HA, Suls JM, editors. Handbook of Psychology. 2nd edn. Indianapolis, IN: Wiley-Blackwell Press; 2012. pp. 391–406. [Google Scholar]
- Sheppes G, Levin Z. Emotion regulation choice: selecting between cognitive regulation strategies to control emotion. Frontiers in Human Neuroscience. 2013;7:179. doi: 10.3389/fnhum.2013.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppes G, Meiran N. Better late than never? On the dynamics of online regulation of sadness using distraction and cognitive reappraisal. Personality and Social Psychology Bulletin. 2007;33(11):1518–32. doi: 10.1177/0146167207305537. [DOI] [PubMed] [Google Scholar]
- Sheppes G, Meiran N. Divergent cognitive costs for online forms of reappraisal and distraction. Emotion. 2008;8(6):870–4. doi: 10.1037/a0013711. [DOI] [PubMed] [Google Scholar]
- Sheppes G, Scheibe S, Suri G, Gross JJ. Emotion-regulation choice. Psychological Science. 2011;22(11):1391–6. doi: 10.1177/0956797611418350. [DOI] [PubMed] [Google Scholar]
- Sheppes G, Scheibe S, Suri G, Radu P, Blechert J, Gross JJ. Emotion regulation choice: a conceptual framework and supporting evidence. Journal of Experimental Psychology: General. 2014a;143(1):163–81. doi: 10.1037/a0030831. [DOI] [PubMed] [Google Scholar]
- Sheppes G, Suri G, Gross JJ. Emotion regulation and psychopathology. Annual Review of Clinical Psychology. 2015;11:379–405. doi: 10.1146/annurev-clinpsy-032814-112739. [DOI] [PubMed] [Google Scholar]
- Silvers JA, Weber J, Wager TD, Ochsner KN. Bad and worse: neural systems underlying reappraisal of high- and low-intensity negative emotions. Social Cognitive and Affective Neuroscience. 2015;10(2):172–9. doi: 10.1093/scan/nsu043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiruchselvam R, Blechert J, Sheppes G, Rydstrom A, Gross JJ. The temporal dynamics of emotion regulation: an EEG study of distraction and reappraisal. Biological Psychology. 2011;87(1):84–92. doi: 10.1016/j.biopsycho.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Wessa M, Kanske P, Neumeister P, Bode K, Heissler J, Schönfelder S. EmoPics: Subjektive und psychophysiologische Evaluationen neuen Bildmaterials für die klinisch-biopsychologische Forschung. 2010 [EmoPicS: subjective and psychophysiological evaluation of new imagery for clinical biopsychological research]. Zeitschrift für Klinische Psychologie und Psychotherapie, 39(Suppl. 1/11), 77. [Google Scholar]
- Wilson TD, Gilbert DT. Explaining away: a model of affective adaptation. Perspectives on Psychological Science. 2008;3(5):370–86. doi: 10.1111/j.1745-6924.2008.00085.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.