Abstract
Respiratory viruses are the leading cause of acute infections in humans. However, the burden of certain respiratory viruses, such as coronaviruses, and the relevance of viral coinfections remain unclear. In this study, we investigated the distribution and seasonal occurrences of respiratory viruses detected by multiplex molecular assay in 6,014 samples from 2008 to 2011 in a French hospital. We assessed the detection frequencies of 14 respiratory viruses and their clinical impact in immunosuppressed and nonimmunosuppressed patients. Furthermore, we explored the preferential association patterns between respiratory viruses in multiple infections. Our results indicated that human rhinovirus/enterovirus (HRV/EV) and coronavirus (HCoV) were frequently detected in respiratory samples (48.81% and 11.74% of infected samples, respectively), and the detection frequencies of these viruses were further increased in immunosuppressed patients. The most common subtypes of HCoV were HCoV-229E (33.80%) and HCoV-HKU1 (32.39%). A sharp increase in the detection frequencies of HCoV-229E and HCoV-HKU1 over several months suggested that these subtypes were epidemic in our population. In immunosuppressed patients, HCoV contributed to upper respiratory tract infections (52%). Evidence did not support lower respiratory tract infections exclusive to a unique HCoV infection. In multiply infected individuals, determined in 6.3% of samples, HRV/EV and HCoV were detected in 33.29% and 22.90% of samples, respectively. Interestingly, nearly 50% of HCoV infections were detected in association with another virus. Since the distributions of respiratory viruses in multiply infected patients were subject to preferential association patterns between viruses, we propose complex interactions between different respiratory viruses and host factors.
INTRODUCTION
Infections of the respiratory tract are the most common types of acute infections in children and adults (1). It has been estimated that young children have on average 6 to 8 respiratory viral infections per year, while adults have 2 to 4 infections per year (2). Viruses account for a large number of these respiratory infections, which can be divided into upper respiratory tract infections (URTI) and lower respiratory tract infections (LRTI), albeit this division still represents heterogeneous groups of diseases caused by numerous viruses (1). Viral infections of the lower respiratory tract cause a dramatic disease burden, especially among the more vulnerable subjects, such as young children, the elderly, and immunocompromised patients (1, 3–5).
The recent development of multiplex PCR assays has improved the detection of respiratory viruses (6, 7), since these molecular techniques are rapid, sensitive, and can be performed on a wide variety of respiratory samples. Multiplex PCR assays allow identification of a large panel of respiratory viruses, including influenza virus A and B, parainfluenza viruses (PIV) 1 to 4, respiratory syncytial virus (RSV), and newly discovered viruses not detected by routine clinical tests, including coronavirus (HCoV) subtype HKU1 (8), HCoV-NL63 (9), and human metapneumovirus (HMPV) (10). In clinical practice, the rapid detection of respiratory viruses might be of great interest for avoiding empirical antibiotic treatments, starting adapted therapies, or in taking preventive measures to limit the disease spread (6, 7). However, an ongoing challenge has been that the epidemiology and the distinct contributions of certain respiratory viruses to the overall health burden remain unclear. The viruses that likely contribute to general respiratory infections include the “classical” subtypes of coronaviruses, i.e., HCoV-229E, -OC43, -HKU1, and -NL63. The contribution of these viruses has not been fully evaluated, either in the general population or among the more vulnerable immunosuppressed patients. Furthermore, the interference between viruses in the respiratory tract and the clinical impact of respiratory viral coinfections remain poorly understood (11–13).
In this study, we comprehensively investigated the distribution of respiratory viruses in samples from immunosuppressed and nonimmunosuppressed patients tested by multiplex PCR assay in our institution. We especially noticed the high incidence of coronaviruses, their epidemic potential, and their low impact on respiratory health, even in immunosuppressed patients. By characterizing the virome from patients with multiple respiratory viral infections, we also observed the preferential associations that occurred between viruses in the respiratory tract.
MATERIALS AND METHODS
Clinical samples.
From January 2008 to December 2011, 6,014 respiratory specimens from 4,119 patients were collected for routine molecular diagnostics at the Department of Virology, Strasbourg University Hospital, Strasbourg, France. Samples were obtained from inpatients in Strasbourg University Hospital and from outpatients of the French region Alsace with the following distribution: 22.3% from outpatients of the Regional Group of Influenza Observation (GROG), a French network of private practice general practitioners and pediatricians dedicated to the virological surveillance of influenza, 30.2% from the Pediatric Department, 18.3% from the Pneumology Department, 7.4% from medical intensive care units, 6.9% from the Adult Oncology-Hematology Department, and 4.3% from surgery and surgical intensive care units. Additional specimens were obtained either from mixed medical departments of Strasbourg University Hospital or from other regional institutions, including regional public hospitals and private practice physicians (10.6%). Samples included were nasopharyngeal swabs (Sigma Virocult, Medical Wire and Equipment, Corsham, Wiltshire, England; n = 2,953), bronchoalveolar lavage fluids (n = 1,348), nasopharyngeal aspirates (n = 947), oropharyngeal swabs (Sigma Virocult, Medical Wire and Equipment; n = 442), tracheal aspirates (n = 152), sputum samples (n = 89), and pleural effusion (n = 59) and pulmonary biopsy (n = 24) specimens. Samples were transported to the laboratory within 4 h and stored at −80°C until nucleic acid extraction and subsequent PCR analysis. Data were analyzed retrospectively.
Clinical data.
Patient information, including age, sex, place of sample collection, and type of respiratory sample, was recorded in the setting of routine molecular diagnostics. For HCoV-infected immunosuppressed patients, additional data, including respiratory and extrarespiratory symptoms, disease outcome, type of immunosuppression, and associated respiratory pathogens (virus, bacteria, and fungi), were retrospectively obtained from medical charts by using a standardized form and supplemented by analyzing the local laboratory information management system. For each HCoV-positive sample, the presence of an associated respiratory pathogen was taken into account if its detection occurred during the interval from study day −10 to day +10. Similarly, respiratory symptoms were considered when they occurred during the interval 10 days before or after HCoV detection. Respiratory symptoms were assigned to lower respiratory tract infections in the presence of bronchitis, pneumonia, bronchiolitis, dyspnea, acute respiratory distress syndrome, wheezing, or indicative chest X-ray abnormalities. Rhinopharyngitis/rhinorrhoea, tonsillitis, and sore throat in the absence of any sign of LRTI were classified as URTI. All samples were collected and tested via methods that conformed to the ethical legislation in place.
Nucleic acid extraction and respiratory virus detection.
Nucleic acid extraction was performed using the QIAamp viral RNA minikit (Qiagen, Valencia, CA) according to the manufacturer's instructions. Nucleic acids were extracted from 200 μl of sample and eluted in 55 μl of elution buffer. Respiratory specimens were tested using the xTAG RVP Fast kit (Luminex, Austin, TX) on a Luminex 200 system analyzer according to the manufacturer's instructions. Fourteen respiratory viruses were detectable by this technique: adenovirus (AdV), human rhinovirus/enterovirus (HRV/EV), HCoV-229E, HCoV-HKU1, HCoV-OC43, HCoV-NL63, HMPV, influenza virus types A and B, PIV 1 to 4, and RSV.
Statistical analysis.
Statistical analysis for comparison of proportions was performed using OpenStat software, and differences were considered significant at a P value of <0.05. Where viral codetections occurred, associations between viruses were assessed using a two-by-two Pearson's chi-square analysis with the Yates correction using R software (http://www.r-project.org). In cases where Pearson's chi-square analysis was constrained by low expected values, Fischer's exact test was employed. Odds ratio (ORs) and 95% confidence interval (CIs) for the likelihood of codetection were calculated using R software.
RESULTS
Distribution of viruses in respiratory samples.
To evaluate the distribution of respiratory viruses detected in our institution, we retrospectively analyzed 6,014 respiratory samples collected for routine molecular diagnostics over a 4-year period (2008 to 2011) and screened the samples for 14 respiratory viruses by using a commercially available multiplex PCR assay. The median age of patients was 20.1 years (range, 1 day to 93.9 years).
Among all collected samples, 37.4% were infected by one virus, 6.1% were simultaneously infected by 2 viruses, and 0.2% by 3 different viruses. No respiratory virus was detectable in the remaining 56.3% of specimens. HRV/EV were the most frequently detected viruses (48.81%) among overall positive respiratory samples (Table 1), followed by RSV (12.30%) and coronaviruses (HCoV-229E, -OC43, -NL63, and -HKU1; 11.74%). Less frequently detected respiratory viruses were influenza virus A and B, HMPV, AdV, and PIV 1 to 4 (Table 1). The distribution of detected respiratory viruses by sampling method is described in Table S1 of the supplemental material.
Table 1.
Detection frequencies of respiratory viruses over the 4-year study period
| Respiratory virus | No. of positive samples (%) in patient groupa | P valueb | ||
|---|---|---|---|---|
| All | Immunosuppressed | Nonimmunosuppressed | ||
| HRV/EV | 1,476 (48.81) | 214 (59.12) | 1,247 (47.45) | <0.0001 |
| RSV | 372 (12.30) | 37 (10.22) | 331 (12.60) | NS |
| HCoV | 355 (11.74) | 56 (15.47) | 291 (11.07) | 0.01 |
| Influenza virus A | 187 (6.18) | 12 (3.31) | 174 (6.62) | 0.01 |
| HMPV | 158 (5.22) | 11 (3.04) | 145 (5.52) | 0.05 |
| AdV | 148 (4.89) | 9 (2.49) | 137 (5.21) | 0.02 |
| Influenza virus B | 126 (4.17) | 4 (1.10) | 122 (4.64) | 0.002 |
| PIV 3 | 72 (2.38) | 10 (2.76) | 62 (2.36) | NS |
| PIV 1 | 46 (1.52) | 2 (0.55) | 43 (1.64) | NS |
| PIV 4 | 44 (1.46) | 2 (0.55) | 41 (1.56) | NS |
| PIV 2 | 40 (1.32) | 5 (1.38) | 35 (1.33) | NS |
| Total | 3,024 (100) | 362 (100) | 2,628 (100) | |
Data include all respiratory viruses detected in monoinfected and multiply infected samples. The number of samples included in the immunosuppressed and nonimmunosuppressed groups was 831 and 5,078, respectively.
P value for the comparison of viral detection frequencies between the immunosuppressed and nonimmunosuppressed groups. NS, not significant.
Because the burden of respiratory viruses may be more important in immunosuppressed patients, we selected a restricted group of 831 respiratory samples sent from adult and pediatric onco-hematology departments and posttransplantation departments. The median age in this group was 22.8 years. Data obtained from this immunosuppressed group of samples were compared to those for a nonimmunosuppressed group composed of the remaining specimens collected in patients for whom we had no specific clinical notion of immunosuppression (n = 5,078). The percentage of positive samples was lower in the immunosuppressed patients (38.0% versus 45.0%; P = 0.0002), and the percentage of samples containing one virus was also lower (32.7% versus 38.5%; P = 0.0014). There was no significant difference in the detection rates of 2 or 3 viruses between the two groups (5.1% versus 6.3% for 2 viruses, P = 0.18; 0.2% versus 0.2% for 3 viruses, P = 0.98).
Interestingly, the frequencies of HRV/EV and HCoV were significantly higher in immunosuppressed patients than in nonimmunosuppressed patients (P < 0.0001 and P = 0.01, respectively), while HCoVs were the second most commonly detected viruses in immunosuppressed patients (Table 1).
Distribution and clinical impact of coronaviruses.
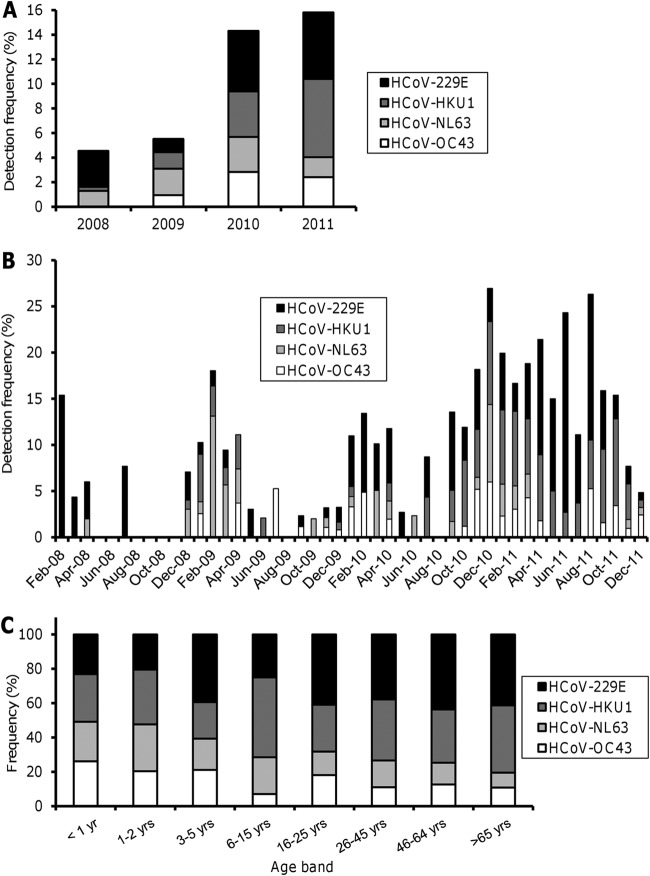
Since HCoVs were frequently detected in our population, we investigated the distribution of each subtype of HCoV over the 4 years of the study. HCoV-229E and HCoV-HKU1 were the most frequently detected subtypes among overall samples, followed by HCoV-NL63 and HCoV-OC43 (Table 2). Interestingly, by investigating the yearly change in distribution of each subtype of HCoV, we observed a sharp increase in the detection frequencies of HCoV-229E and HCoV-HKU1 (and to a lesser extent HCoV-OC43) in 2010 and 2011 relative to 2008 and 2009 (Fig. 1A). Whereas HCoV predominated during the winters of 2008, 2009, and 2010, we noticed that HCoV-229E and HCoV-HKU1 still represented a significant proportion of overall detected viruses during the spring and summer of 2011 (Fig. 1B). Taken together, these data suggest a persistent epidemic of HCoV-229E and HCoV-HKU1 during several months in 2010 and 2011 in our population. Since the subtypes HCoV-OC43 and HCoV-NL63 have been described to predominate in children (14–18), we investigated the distribution of each subtype of HCoV according to age. HCoV-OC43 and HCoV-NL63 were more frequently detected in children less than 2 years old than in older children and in adults. Conversely, HCoV-229E and HCoV-HKU1 were the most frequently observed coronaviruses in children above 3 years and in adults, although some variation occurred in the percentage of each subtype between the different age groups (Fig. 1C).
Table 2.
Detection frequencies of HCoV subtypes over the 4-year study period
| Respiratory virus | No. of positive samples (%) in patient groupa | P valueb | ||
|---|---|---|---|---|
| All | Immunosuppressed | Nonimmunosuppressed | ||
| HCoV-229E | 120 (33.80) | 21 (37.50) | 95 (32.65) | NS |
| HCoV-HKU1 | 115 (32.39) | 17 (30.36) | 96 (32.99) | NS |
| HCoV-NL63 | 62 (17.46) | 15 (26.79) | 45 (15.46) | 0.04 |
| HCoV-OC43 | 58 (16.34) | 3 (5.36) | 55 (18.90) | 0.01 |
| Total | 355 (100) | 56 (100) | 291 (100) | |
The number of samples included in the immunosuppressed and nonimmunosuppressed groups was 831 and 5,078 respectively.
P value for the comparison of viral detection frequencies between the immunosuppressed and nonimmunosuppressed groups. NS, not significant.
Fig 1.
Distribution of HCoV between January 2008 and December 2011. (A) Detection frequencies of HCoV-229E, -HKU1, -NL63, and -OC43 by year. Values represent the percentage of each subtype of HCoV among overall positive samples for each year. (B) Seasonal distribution of HCoV. Data represent the percentage of each subtype of HCoV among overall positive samples for each month. (C) Distribution of the four subtypes of HCoV by age group.
There was no significant difference in the detection frequencies of HCoV-229E and HCoV-HKU1 between immunosuppressed and nonimmunosuppressed patients. In contrast, among the two less frequently detected subtypes of HCoV, the frequency of HCoV-NL63 was significantly higher in immunosuppressed patients (P = 0.04), whereas HCoV-OC43 predominated in nonimmunosuppressed patients (P = 0.01) (Table 2).
The impact on respiratory health due to HCoV infection of immunosuppressed patients remains poorly understood. To assess the clinical impact of HCoV infections in immunosuppressed patients, we investigated 50 samples positively identified for HCoV infection from 39 patients. In all patients, HCoV infection occurred during a well-documented episode of immunosuppression. Acute URTI and LRTI were documented in 52% (n = 26) and 28% (n = 14) of samples, respectively. The 20% (n = 10) of remaining HCoV-positive samples were obtained either during prolonged cough investigations or from systematic solid organ transplantation follow-up, without specific acute URTI or LRTI symptoms (Table 3). URTI was the most common clinical presentation during infections by the subtypes HCoV-229E, -HKU1, -OC43, and -NL63. Rhinopharyngitis/rhinorrhea was the most frequently observed symptom during HCoV infections in immunocompromised patients (58% of cases), followed by cough (46%) and fever (28%). Pneumonia was diagnosed in 14% of patients (Table 3). By focusing on patients with LRTI, we noticed that HCoV detection was associated either with the detection of other viral or nonviral respiratory pathogens (including bacteria, such as Pseudomonas aeruginosa [colonization] and Klebsiella pneumoniae, or fungi, such as Aspergillus sp. and Pneumocystis jirovecii) and/or with noninfectious respiratory diseases that might partially account for symptoms such as pulmonary embolism, bronchiolitis obliterans syndrome, bronchiectasis, and pleuresia. Associations observed between HCoV and nonviral pathogens in the investigated group of immunosuppressed patients (with URTI or LRTI) are described in Table S2 of the supplemental material. In contrast, we failed to observe any episode of LRTI in immunosuppressed patients that was exclusively explained by a single HCoV infection (Table 4). Conversely, HCoV was the only detected pathogen in 58% of URTI in immunosuppressed patients (Table 4). Taken together, these results suggest that HCoV infection in immunosuppressed patients predominately results in URTI. Although the coronavirus contribution to LRTI cannot be excluded, our data suggest that associated pathogens and noninfectious respiratory diseases should be investigated before attributing lower respiratory symptoms to an HCoV infection.
Table 3.
Clinical presentation of HCoV infections in immunosuppressed patients
| Clinical manifestation | No. (%) of positive samples found in HCoV-infected patients with symptom | ||||
|---|---|---|---|---|---|
| Any HCoV (n = 50) | HCoV-229 (n = 19) | HCoV-HKU1 (n = 17) | HCoV-OC43 (n = 4) | HCoV-NL63 (n = 13) | |
| URTI | 26 (52.0) | 8 (42.1) | 8 (47.1) | 2 (50.0) | 9 (69.2) |
| LRTI | 14 (28.0) | 7 (36.8) | 4 (23.5) | 1 (25.0) | 2 (15.4) |
| Prolonged cough investigation/posttransplantation follow-up | 10 (20.0) | 4 (21.1) | 5 (29.4) | 1 (25.0) | 2 (15.4) |
| Rhinopharyngitis/rhinorrhea | 29 (58.0) | 9 (47.4) | 9 (52.9) | 2 (50.0) | 10 (76.9) |
| Cough | 23 (46.0) | 7 (36.8) | 12 (70.6) | 2 (50.0) | 4 (30.8) |
| Fever of >38°C | 14 (28.0) | 9 (47.4) | 3 (17.6) | 0 (0.0) | 4 (30.8) |
| Pneumonia | 7 (14.0) | 5 (26.3) | 1 (5.9) | 1 (25.0) | 0 (0.0) |
| Dyspnea | 3 (6.0) | 2 (10.5) | 0 (0.0) | 0 (0.0) | 1 (7.7) |
| Acute bronchitis | 3 (6.0) | 1 (5.3) | 2 (11.8) | 0 (0.0) | 0 (0.0) |
| Acute respiratory distress/intensive care unit | 2 (4.0) | 1 (5.3) | 0 (0.0) | 0 (0.0) | 1 (7.7) |
| Bronchiolitis | 1 (2.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (7.7) |
| Death | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Table 4.
Associated pathogens and noninfectious respiratory diseases in HCoV-infected immunosuppressed patients
| Clinical and microbiological context | % of patients presenting with: | |
|---|---|---|
| LRTI (n = 14) | URTI (n = 26) | |
| Presence of associated respiratory virus | 71 | 38 |
| Presence of associated nonviral respiratory pathogena | 43 | 4 |
| Noninfectious respiratory disease at presentationa | 29 | 0 |
| Only HCoVb | 0 | 58 |
Present at the time of HCoV detection and which could at least partially explain the symptoms.
No detection of an associated viral or nonviral pathogen and absence of diagnosed noninfectious respiratory disease.
Coinfection by respiratory viruses.
A total of 379 (6.3%) respiratory samples were shown to harbor more than one virus (6.1% samples were infected by two viruses and 0.2% by three viruses). Analysis of these multiply infected specimens demonstrated that HRV/EV, HCoV, and RSV were the most frequently observed viruses in total collected samples (Table 5). The HCoV frequency was significantly higher in immunosuppressed patients than in nonimmunosuppressed patients (P = 0.002 [Table 5]). Interestingly, we noticed that 49.8% (177/355) of all detected HCoV was in coinfected samples, and this proportion reached 57.1% (32/56) when we restricted the analysis to immunosuppressed patients, indicating that HCoV infections are frequently associated with other respiratory viruses.
Table 5.
Detection frequencies of respiratory viruses in multiply infected samples over the 4-year study period
| Respiratory virus | No. of positive samples (%) in patient groupa | P valueb | ||
|---|---|---|---|---|
| All | Immunosuppressed | Nonimmunosuppressed | ||
| HRV/EV | 257 (33.29) | 37 (41.11) | 218 (32.44) | NS |
| HCoV | 177 (22.90) | 32 (35.56) | 141 (20.98) | 0.002 |
| RSV | 105 (13.60) | 6 (6.67) | 98 (14.58) | 0.04 |
| AdV | 75 (9.72) | 4 (4.44) | 69 (10.27) | NS |
| HMPV | 41 (5.31) | 3 (3.33) | 38 (5.65) | NS |
| Influenza virus A | 31 (4.02) | 0 (0.00) | 31 (4.61) | 0.04 |
| PIV 3 | 26 (3.37) | 3 (3.33) | 23 (3.42) | NS |
| Influenza virus B | 24 (3.11) | 1 (1.11) | 23 (3.42) | NS |
| PIV 1 | 18 (2.33) | 1 (1.11) | 16 (2.38) | NS |
| PIV 4 | 10 (1.30) | 0 (0.00) | 10 (1.49) | NS |
| PIV 2 | 8 (1.04) | 3 (3.33) | 5 (0.74) | 0.02 |
| Total | 772 (100) | 90 (100) | 672 (100) | |
The number of samples included in the immunosuppressed and nonimmunosuppressed groups was 831 and 5,078 respectively.
P value for the comparison of viral detection frequencies between immunosuppressed and nonimmunosuppressed groups. NS, not significant.
We next investigated whether the distribution of respiratory viruses in dual-infected samples was random or if there were preferential associations between viruses. A total of 365 dual-infected specimens was assessed using univariate analysis. Among the four most frequently detected viruses in coinfected samples (HRV/EV, HCoV, RSV, and AdV), we observed a unique and highly significant positive association between HCoV-229E and HCoV-HKU1 (OR, 3.50; 95% CI, 1.75 to 6.89; P = 0.0002), whereas both HCoV-229E and HCoV-HKU1 were less frequently detected in the presence of the two other subtypes of HCoV, HCoV-NL63 and HCoV-OC43 (Table 6). The presence of HCoV-229E or HCoV-HKU1 decreased the odds of detecting AdV, HRV/EV, or RSV (Table 6). Similarly, AdV, HRV/EV, and RSV were less frequently detected in the presence of the other common respiratory viruses mentioned in Table 6. Although each respiratory virus exhibited different association patterns, most of the investigated viruses reduced the probability of codetection of one or more associated respiratory viruses.
Table 6.
Measures of association between respiratory virus pairs in coinfected samples determined using two-by-two contingency tables
| Virus | P value (OR) for association with paired virus in coinfected samplesa | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HCoV-229E | HCoV-HKU1 | HCoV-NL63 | HCoV-OC43 | HRV/EV | Influenza virus A | Influenza virus B | HMPV | PIV-1 | PIV-2 | PIV-3 | PIV-4 | RSV | |
| AdV | 0.04 (0.34) | 0.001 (0.12) | 0.61 (0.97) | 0.03 (0.17) | 0.16 (0.66) | 0.02 (0.14) | 0.01 (0) | 0.03 (0.20) | 0.07 (2.62) | 0.52 (0.59) | 0.007 (0) | 0.518 (0.59) | <0.0001 (0.16) |
| HCoV-229E | 0.0002 (3.50) | 0.03 (0) | 0.02 (0) | <0.0001 (0.16) | 0.79 (1.11) | 0.55 (0.51) | 0.09 (0.26) | 0.06 (0) | 0.63 (0.75) | 0.02 (0) | 0.25 (0) | 0.0002 (0.16) | |
| HCoV-HKU1 | 0.03 (0) | 0.02 (0) | <0.0001 (0.18) | 0.19 (0.36) | 0.15 (0.23) | 0.18 (0.39) | 0.49 (0.72) | 0.61 (0.72) | 0.07 (0.21) | 0.23 (0) | 0.004 (0.30) | ||
| HCoV-NL63 | 0.23 (0) | 0.06 (0.40) | 1.00 (0.56) | 0.63 (0.23) | 0.09 (0) | 0.38 (0) | 0.62 (0) | 0.23 (0) | 0.62 (0) | 0.88 (1.05) | |||
| HCoV-OC43 | 0.08 (0.44) | 1.00 (1.06) | 1.00 (0.66) | 0.48 (1.21) | 0.33 (0) | 0.58 (0) | 0.52 (0.60) | 0.58 (0) | 0.31 (0.50) | ||||
| HRV/EV | 0.007 (0.34) | 0.09 (0.44) | 0.06 (0.50) | 0.17 (0.45) | 0.71 (0.77) | 0.116 (2.44) | 0.44 (3.32) | 0.02 (0.55) | |||||
| Influenza virus A | 0.24 (0) | 0.75 (0.60) | 0.63 (0.60) | 1.00 (0) | 0.24 (0) | 1.00 (0) | 0.03 (0.28) | ||||||
| Influenza virus B | 0.49 (0.38) | 0.61 (0.38) | 1.00 (0) | 0.38 (0) | 1.00 (0) | 1.00 (0.98) | |||||||
| HMPV | 0.16 (0) | 0.40 (0) | 0.06 (0) | 0.40 (0) | 0.04 (0.35) | ||||||||
| PIV-1 | 0.70 (0) | 0.33 (0) | 0.70 (0) | 0.008 (0) | |||||||||
| PIV-2 | 0.58 (0) | 0.84 (0) | 0.07 (0) | ||||||||||
| PIV-3 | 0.58 (0) | 0.05 (0.22) | |||||||||||
| PIV-4 | 0.07 (0) | ||||||||||||
Probabilities of association (P values) are shown for each pair, with the odds ratio in parentheses. P values indicating significant associations (P < 0.05) are shown in bold.
DISCUSSION
The comprehensive analysis of respiratory samples detected by multiplex molecular assay in our institution between 2008 and 2011 revealed that the most frequently detected viruses were HRV/EV, RSV, and HCoV. These viruses were also the most common when we restricted the analysis to immunosuppressed patients.
HRV/EV are increasingly regarded as significant respiratory pathogens in both upper and lower respiratory tract infections (1, 19–23). In contrast, the distribution and the clinical impact of the “classical” subtypes of HCoV remain unclear (“classical” subtypes refer to the four subtypes HCoV-229E, -OC43, -HKU1, and -NL63 but exclude the highly pathogenic severe acute respiratory syndrome HCoV [24] and the recently described subtype Middle East respiratory syndrome CoV [25]). Detection rates of HCoV in respiratory samples reported in the literature are highly variable, ranging from 2% to 13% depending on the country, the studied population, and the modality of detection (15–18, 26–29). Here, we noticed a high detection rate of HCoV in our institution (11.74% of positive samples). In immunosuppressed patients, HCoVs were the second most commonly detected viruses (15.47%), raising the important question of the clinical impact of HCoV in this highly vulnerable group of patients. To address this point, we retrospectively reviewed the clinical presentation of HCoV-positive immunosuppressed patients at the time of viral detection. We observed that HCoV detection was predominantly associated with URTI in immunosuppressed patients. Cough, fever, and rhinopharyngitis/rhinorrhea were the most frequently associated symptoms during HCoV infections, consistent with previous reports (16, 27, 30). However, a significant number of HCoV infections in immunosuppressed patients (28% of cases) were also observed in association with clinical manifestations of LRTI, such as pneumonia, acute bronchitis, or dyspnea. In all the cases of LRTI in HCoV-infected patients, associated viral or nonviral pathogens and/or noninfectious respiratory diseases were diagnosed and could have accounted for the symptoms. In contrast, we failed to observe any episode of LRTI that could exclusively be explained by a monoinfection of HCoV. Although the possible involvement of the “classical” subtypes of HCoV in LRTI has been suggested in children and adults (26, 28, 31–33), our findings challenge the clinical impact of these viruses in immunosuppressed patients. Our results indicate that other possible associated respiratory pathogens and noninfectious respiratory diseases should be investigated before attributing the unique etiology of an LRTI to a “classical” coronavirus.
The most commonly observed subtypes of HCoV in our survey were HCoV-229E and HCoV-HKU1. A higher incidence of these two subtypes was predominate during several months in 2010 and to a greater extent in 2011, suggesting a sustained epidemic of these strains at that time in our population. Interestingly, although the distributions of the four classical subtypes of HCoV have been described to vary by year and location (15, 17, 18, 26, 27, 29, 34), many previous studies (mostly performed in children [14–18]) have reported a predominance of the two subtypes HCoV-OC43 and HCoV-NL63 (14–18). In a recent longitudinal survey carried out with children less than 2 years old, Dijkman et al. noticed that HCoV-NL63 and HCoV-OC43 infections occurred more frequently in early childhood than infections due to the HCoV-229E and HCoV-HKU1 subtypes (14), and the authors suggested that HCoV-OC43 and HCoV-NL63 might elicit a protective immunity against the subsequent infections by HCoV-HKU1 and HCoV-229E, respectively. Similarly, higher prevalences of HCoV-OC43 and HCoV-NL63 were observed in children less than 2 years old in our study, whereas HCoV-229E and HCoV-HKU1 predominated in children older than 3 years old and in adults, providing strong evidence that the distributions of the classical subtypes of HCoV vary between age groups. A lack of cross-protective immunity between HCoV-229E and HCoV-HKU1 (which belong to two different genera, Alphacoronavirus and Betacoronavirus, respectively [35]), might account for their simultaneous epidemic potentials.
Analysis of dual-infected samples provided statistical evidence that coinfections by respiratory viruses are not random. A unique preferential codetection was observed between HCoV-229E and HCoV-HKU1. For all other statistically significant pairs of viruses, viral cosuppression (where infection by one virus reduced the risk for infection by the other virus) was common. Similar patterns of preferential viral associations in the respiratory tract have previously been described (11–13). It remains unclear whether these associations correlate with overlap of epidemic seasons, as would be expected for the highly significant positive association we observed between HCoV-229 and HCoV-HKU1, or whether these associations are sustained by pathophysiological phenomena. One previously formulated hypothesis was that the activation of intrinsic immune defenses during the occurrence of a first viral infection subsequently induces an antiviral state that shields neighboring cells from a subsequent viral infection during a refractory period (13). Such interference was notably attributed to double-stranded RNA (dsRNA) generated during the replication of RNA viruses that might trigger interferon-mediated innate immunity (11, 13). However, using a comprehensively tested collection of specimens, we also noticed a broad pattern of negative associations with AdV (a DNA virus) that was not described in former studies (11–13). The pattern of negative associations with such a DNA virus in our survey argues in favor of a more general activation of viral sensing and innate defenses during respiratory viral infections. Interestingly, the detailed analysis of HCoV subtypes led us to observe variations in the preferential associations between viruses within the HCoV family, suggesting a complex interplay between viruses that may vary even between common viral family members.
This study may have had some limitations. In particular, the decision to perform virological assays for patients with a similar clinical presentation may have varied according to the immune status of patients and to the sampling practice of clinicians. Consequently, a sampling bias in the comparison of detection frequencies of viruses between immunosuppressed and immunocompetent patients cannot be ruled out and warrants further investigation.
In conclusion, our study provides new insight into the distribution patterns of respiratory viruses in both immunosuppressed and nonimmunosuppressed patients. In particular, we highlighted that HCoV may be quite frequently detected in the respiratory tract, notably through epidemic episodes due to one or more subtypes of HCoV, and are commonly associated with other respiratory viruses. The clinical impact of HCoV remained low, even in immunosuppressed patients, suggesting that the involvement of possible associated pathogens or noninfectious respiratory diseases should be ruled out before attributing the unique etiology of an LRTI to HCoV. Finally, the observation that respiratory viruses exhibit different association patterns during multiple infections highlights the complex relationships that might occur between respiratory viruses and their host in the respiratory tract.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grant API HUS 2012/5256 from Hôpitaux Universitaires de Strasbourg.
We thank Audrey Weber, Lola Hamied, and Simone Risch for their technical assistance.
Footnotes
Published ahead of print 12 July 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.01078-13.
REFERENCES
- 1.Gaunt ER, Harvala H, McIntyre C, Templeton KE, Simmonds P. 2011. Disease burden of the most commonly detected respiratory viruses in hospitalized patients calculated using the disability adjusted life year (DALY) model. J. Clin. Virol. 52:215–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heikkinen T, Jarvinen A. 2003. The common cold. Lancet 361:51–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Talbot HK, Falsey AR. 2010. The diagnosis of viral respiratory disease in older adults. Clin. Infect. Dis. 50:747–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chemaly RF, Ghosh S, Bodey GP, Rohatgi N, Safdar A, Keating MJ, Champlin RE, Aguilera EA, Tarrand JJ, Raad II. 2006. Respiratory viral infections in adults with hematologic malignancies and human stem cell transplantation recipients: a retrospective study at a major cancer center. Medicine (Baltimore) 85:278–287 [DOI] [PubMed] [Google Scholar]
- 5.Ruuskanen O, Lahti E, Jennings LC, Murdoch DR. 2011. Viral pneumonia. Lancet 377:1264–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahony JB. 2008. Detection of respiratory viruses by molecular methods. Clin. Microbiol. Rev. 21:716–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caliendo AM. 2011. Multiplex PCR and emerging technologies for the detection of respiratory pathogens. Clin. Infect. Dis. 52(Suppl 4):S326–S330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woo PC, Lau SK, Chu CM, Chan KH, Tsoi HW, Huang Y, Wong BH, Poon RW, Cai JJ, Luk WK, Poon LL, Wong SS, Guan Y, Peiris JS, Yuen KY. 2005. Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J. Virol. 79:884–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Hoek L, Pyrc K, Jebbink MF, Vermeulen-Oost W, Berkhout RJ, Wolthers KC, Wertheim-van Dillen PM, Kaandorp J, Spaargaren J, Berkhout B. 2004. Identification of a new human coronavirus. Nat. Med. 10:368–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van den Hoogen BG, de Jong JC, Groen J, Kuiken T, de Groot R, Fouchier RA, Osterhaus AD. 2001. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat. Med. 7:719–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brunstein JD, Cline CL, McKinney S, Thomas E. 2008. Evidence from multiplex molecular assays for complex multipathogen interactions in acute respiratory infections. J. Clin. Microbiol. 46:97–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanner H, Boxall E, Osman H. 2012. Respiratory viral infections during the 2009–2010 winter season in Central England, UK: incidence and patterns of multiple virus co-infections. Eur. J. Clin. Microbiol. Infect. Dis. 31:3001–3006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greer RM, McErlean P, Arden KE, Faux CE, Nitsche A, Lambert SB, Nissen MD, Sloots TP, Mackay IM. 2009. Do rhinoviruses reduce the probability of viral co-detection during acute respiratory tract infections? J. Clin. Virol. 45:10–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dijkman R, Jebbink MF, Gaunt E, Rossen JW, Templeton KE, Kuijpers TW, van der Hoek L. 2012. The dominance of human coronavirus OC43 and NL63 infections in infants. J. Clin. Virol. 53:135–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaunt ER, Hardie A, Claas EC, Simmonds P, Templeton KE. 2010. Epidemiology and clinical presentations of the four human coronaviruses 229E, HKU1, NL63, and OC43 detected over 3 years using a novel multiplex real-time PCR method. J. Clin. Microbiol. 48:2940–2947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dominguez SR, Robinson CC, Holmes KV. 2009. Detection of four human coronaviruses in respiratory infections in children: a one-year study in Colorado. J. Med. Virol. 81:1597–1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prill MM, Iwane MK, Edwards KM, Williams JV, Weinberg GA, Staat MA, Willby MJ, Talbot HK, Hall CB, Szilagyi PG, Griffin MR, Curns AT, Erdman DD. 2012. Human coronavirus in young children hospitalized for acute respiratory illness and asymptomatic controls. Pediatr. Infect. Dis. J. 31:235–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Talbot HK, Shepherd BE, Crowe JE, Jr, Griffin MR, Edwards KM, Podsiad AB, Tollefson SJ, Wright PF, Williams JV. 2009. The pediatric burden of human coronaviruses evaluated for twenty years. Pediatr. Infect. Dis. J. 28:682–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Messacar K, Robinson CC, Bagdure D, Curtis DJ, Glode MP, Dominguez SR. 2013. Rhino/enteroviruses in hospitalized children: a comparison to influenza viruses. J. Clin. Virol. 56:41–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peltola V, Waris M, Osterback R, Susi P, Hyypia T, Ruuskanen O. 2008. Clinical effects of rhinovirus infections. J. Clin. Virol. 43:411–414 [DOI] [PubMed] [Google Scholar]
- 21.Miller EK, Lu X, Erdman DD, Poehling KA, Zhu Y, Griffin MR, Hartert TV, Anderson LJ, Weinberg GA, Hall CB, Iwane MK, Edwards KM. 2007. Rhinovirus-associated hospitalizations in young children. J. Infect. Dis. 195:773–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheuk DK, Tang IW, Chan KH, Woo PC, Peiris MJ, Chiu SS. 2007. Rhinovirus infection in hospitalized children in Hong Kong: a prospective study. Pediatr. Infect. Dis. J. 26:995–1000 [DOI] [PubMed] [Google Scholar]
- 23.Jennings LC, Anderson TP, Beynon KA, Chua A, Laing RT, Werno AM, Young SA, Chambers ST, Murdoch DR. 2008. Incidence and characteristics of viral community-acquired pneumonia in adults. Thorax 63:42–48 [DOI] [PubMed] [Google Scholar]
- 24.Peiris JS, Guan Y, Yuen KY. 2004. Severe acute respiratory syndrome. Nat. Med. 10:S88–S97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. 2012. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 367:1814–1820 [DOI] [PubMed] [Google Scholar]
- 26.Kuypers J, Martin ET, Heugel J, Wright N, Morrow R, Englund JA. 2007. Clinical disease in children associated with newly described coronavirus subtypes. Pediatrics 119:e70–e76 [DOI] [PubMed] [Google Scholar]
- 27.Gerna G, Percivalle E, Sarasini A, Campanini G, Piralla A, Rovida F, Genini E, Marchi A, Baldanti F. 2007. Human respiratory coronavirus HKU1 versus other coronavirus infections in Italian hospitalised patients. J. Clin. Virol. 38:244–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lieberman D, Shimoni A, Shemer-Avni Y, Keren-Naos A, Shtainberg R. 2010. Respiratory viruses in adults with community-acquired pneumonia. Chest 138:811–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dare RK, Fry AM, Chittaganpitch M, Sawanpanyalert P, Olsen SJ, Erdman DD. 2007. Human coronavirus infections in rural Thailand: a comprehensive study using real-time reverse-transcription polymerase chain reaction assays. J. Infect. Dis. 196:1321–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cui LJ, Zhang C, Zhang T, Lu RJ, Xie ZD, Zhang LL, Liu CY, Zhou WM, Ruan L, Ma XJ, Tan WJ.2011. Human coronaviruses HCoV-NL63 and HCoV-HKU1 in hospitalized children with acute respiratory infections in Beijing, China. Adv. Virol. 2011:129134. 10.1155/2011/129134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gerna G, Campanini G, Rovida F, Percivalle E, Sarasini A, Marchi A, Baldanti F. 2006. Genetic variability of human coronavirus OC43-, 229E-, and NL63-like strains and their association with lower respiratory tract infections of hospitalized infants and immunocompromised patients. J. Med. Virol. 78:938–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Falsey AR, McCann RM, Hall WJ, Criddle MM, Formica MA, Wycoff D, Kolassa JE. 1997. The “common cold” in frail older persons: impact of rhinovirus and coronavirus in a senior daycare center. J. Am. Geriatr. Soc. 45:706–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johansson N, Kalin M, Tiveljung-Lindell A, Giske CG, Hedlund J. 2010. Etiology of community-acquired pneumonia: increased microbiological yield with new diagnostic methods. Clin. Infect. Dis. 50:202–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vabret A, Dina J, Gouarin S, Petitjean J, Tripey V, Brouard J, Freymuth F. 2008. Human (non-severe acute respiratory syndrome) coronavirus infections in hospitalised children in France. J. Paediatr. Child Health 44:176–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woo PC, Lau SK, Huang Y, Yuen KY. 2009. Coronavirus diversity, phylogeny and interspecies jumping. Exp. Biol. Med. (Maywood) 234:1117–1127 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.