Abstract
Mutations in the catalytic subunit of the mitochondrial DNA polymerase γ (POLG) have been found to be an important cause of neurological disease. Recently, we and collaborators reported a new neurodegenerative disorder with autosomal recessive ataxia in four patients homozygous for two amino acid changes in POLG: W748S in cis with E1143G. Here, we studied the frequency of this allele and found it to be among the most common genetic causes of inherited ataxia in Finland. We identified 27 patients with mitochondrial recessive ataxia syndrome (MIRAS) from 15 Finnish families, with a carrier frequency in the general population of 1:125. Since the mutation pair W748S+E1143G has also been described in European patients, we examined the haplotypes of 13 non-Finnish, European patients with the W748S mutation. Haplotype analysis revealed that all the chromosomes carrying these two changes, in patients from Finland, Norway, the United Kingdom, and Belgium, originate from a common ancient founder. In Finland and Norway, long, common, northern haplotypes, outside the core haplotype, could be identified. Despite having identical homozygous mutations, the Finnish patients with this adult- or juvenile-onset disease had surprisingly heterogeneous phenotypes, albeit with a characteristic set of features, including ataxia, peripheral neuropathy, dysarthria, mild cognitive impairment, involuntary movements, psychiatric symptoms, and epileptic seizures. The high carrier frequency in Finland, the high number of patients in Norway, and the ancient European founder chromosome indicate that this newly identified ataxia should be considered in the first-line differential diagnosis of progressive ataxia syndromes.
Introduction
Polymerase γ (POLG) is considered to be the replicative polymerase of mtDNA and may also participate in mtDNA repair (Longley et al. 1998; Kaguni 2004). The functional POLG consists of a catalytic α core and an accessory β subunit. The catalytic subunit (POLGα) contains the polymerase and exonuclease domains and an interdomain region called “the spacer” that is suggested to play a role in template positioning and interaction with the β subunit (Kaguni 2004). Different mutations in the gene encoding the catalytic subunit, POLG1 (MIM 174763), result in a large variety of clinical phenotypes. Dominant and recessive mutations of POLG1 cause progressive external ophthalmoplegia (PEO [MIM 157640]) (Van Goethem et al. 2001; Lamantea et al. 2002; Luoma et al. 2004), characterized by the accumulation of mtDNA deletions in muscle (Zeviani et al. 1989). Recently, POLG1 mutations, especially those affecting its spacer domain, have been found to be a significant cause of inherited neurodegenerative phenotypes, such as sensory ataxic neuropathy, dysarthria (Van Goethem et al. 2003a), myoclonus, seizures (Van Goethem et al. 2003b), ataxia, and parkinsonism (Luoma et al. 2004). Since neurodegeneration is often associated with aging, it is of major interest that inactivation of the POLG proofreading function results in premature aging in mice (Trifunovic et al. 2004). Another, recently reported clinical outcome of POLG1 mutations is Alpers syndrome (Naviaux and Nguyen 2004; Ferrari et al. 2005), characterized by progressive neurological disorder and liver failure.
We and collaborators recently reported POLG1 mutations in a new neurodegenerative disorder with autosomal recessive ataxia in four patients from two unrelated families (Rantamäki et al. 2001; Van Goethem et al. 2004). These patients were homozygous for two amino acid changes in POLG: W748S in cis with E1143G. The latter is considered a polymorphism, occurring in ∼3% of the North American population. However, its functional effect in combination with the W748S mutation is unknown. Our patients had gait ataxia and peripheral sensory neuropathy with CNS features, such as dysarthria, nystagmus, mild cognitive impairment, degeneration of the central sensory pathway, and epileptic seizures. However, typical findings of a mitochondrial disease were lacking, since these patients had no or few abnormal fibers seen by histological examination of muscle, and the Southern blot analysis of their muscle DNA did not show mtDNA deletions (Van Goethem et al. 2004). Similar findings were described for two Norwegian patients carrying the W748S and E1143G combination (Winterthun et al. 2005). A closely resembling phenotype was also reported in patients homozygous for another spacer domain mutation, A467T (Van Goethem et al. 2003b, 2004; Winterthun et al. 2005), and in patients compound heterozygous for the above-mentioned three mutations (Van Goethem et al. 2004).
We studied the frequency of the W748S mutation in Finnish patients with ataxia, as well as in the Finnish population. We discovered that the mutation is among the most common genetic causes of inherited ataxia in Finland and that the carrier frequency in Finns is high. This prompted a detailed phenotypic study of patients with identical mutations, as well as a genealogical study of Finnish and other European patients with the combination of W748S+E1143G. We present here evidence of a pan-European ancestral mutation for this mitochondrial recessive ataxia syndrome (MIRAS) and suggest that it is a common cause of recessive ataxia in the European population.
Material and Methods
Subjects
This study was approved by the ethical review boards of Turku University and Helsinki University Central Hospital, and informed consent for sample collection and DNA analysis was obtained from the patients. We performed the initial mutation screening by using the sample collection of the University of Turku, which serves as the only diagnostic center in Finland for spinocerebellar ataxia (SCA) mutations. From December 1993 to May 2004, SCA diagnostic samples from 325 patients were sent to this diagnostic center, and 306 of these patients remained without definite SCA diagnosis after mutation screening (for 251 samples, all of the following mutations were screened: SCA1, 2, 3, 6, 7, 8, 10, 12, and 17; DRPLA; and FRDA [MIMs 164400, 183090, 109150, 183086, 164500, 603680, 603516, 604326, 607136, 607462, and 229300, respectively]). Our patients consisted of these 306 undiagnosed patients with various ataxia phenotypes resembling SCAs. Twenty-five patients with SCA8 mutations (nine of these were dominant) were included because SCA8 repeat expansions are present in healthy controls as well, and thus a positive finding is not enough for a definitive SCA8 diagnosis. After the initial screen, local materials from three Finnish university hospitals were screened, and samples from patients with conditions resembling the previously identified patients with the POLG W748S mutation were analyzed. We included in the present study two previously reported Finnish families (Rantamäki et al. 2001; Van Goethem et al. 2004) with members homozygous for the W748S+E1143G mutations. The British patient we included, who was compound heterozygous for the W748S+E1143G and the A467T POLG mutations, has been described elsewhere (Van Goethem et al. 2004). Also part of the present study were two previously unpublished Belgian patients, who were also compound heterozygous for the same mutations. Of the 10 Norwegian patients analyzed, 2 were described elsewhere (Winterthun et al. 2005); the others were unpublished. Four of the Norwegian patients were compound heterozygous for the W748S+E1143G and the A467T POLG mutations, and the remainder were homozygous for the W748S+E1143G POLG mutations.
We gathered medical and family histories of the Finnish patients homozygous for the W748S mutation and performed a genealogical study in accordance with published criteria (Varilo 1999). We traced ancestors back to 1850 from Finnish population registers and local church records and marked the birthplaces of the grandparents on a map of Finland.
To determine the carrier frequency of the W748S mutation in the Finnish population, control individuals were screened for this mutation. Anonymous DNA samples were collected from 150 healthy Red Cross blood donors. Of these controls, 50 were from the province of North Karelia, 50 were from western Finland, and 50 were from the province of Häme. In addition, 10 pooled samples of genomic DNA from 10 control individuals, collected from anonymous Red Cross blood donors, were analyzed by solid-phase minisequencing.
To obtain the incidence of this disease in Finland, the number of patients born in the 1950s (11 patients) was divided by the total number of Finns born in the 1950s (896,175 persons). The corresponding carrier frequency was calculated according to Hardy-Weinberg equilibrium, under the assumption of random mating and nonselection.
DNA Extraction
Genomic DNA was extracted from peripheral blood samples by routine methods. A deparaffinized histological sample lysate was used to analyze the presence of the mutation in one patient, as described elsewhere (Shibata et al. 1988).
PCR and Solid-Phase Minisequencing Analysis
The presence of the p.W748S (c.2243G→C nucleotide change in exon 13 of POLG1 [GenBank accession number NM_002693; sequence numbering starts from ATG]) (Van Goethem et al. 2004) and p.E1143G (c.3428A→G in exon 21) mutations were analyzed by PCR and solid-phase minisequencing (Syvänen et al. 1990). The region of interest was first amplified by PCR with the use of a 5′-biotinylated primer and a 3′-nonbiotinylated primer. The primer sequences for c.2243G→C were 5′-BioCCATGGCAATGGACCTTA-3′ and 5′-GAAACACCACAGGACAGGC-3′, and the primer sequences for c.3428A→G were 5′-Bio-TTTCACCTCTGCCCACCTTC-3′ and 5′-TCAAGAGGTTGGTGATCTGC-3′. PCR was performed in 0.2 mM dNTPs; 1 μM and 0.2 μM of the nonbiotinylated and biotinylated PCR primers, respectively; and 1 U of thermostable DNA polymerase (AmpliTaq Gold DNA Polymerase [Applied Biosystems]), in 50 μl of buffer. The PCR program for c.2243G→C included an initial cycle at 94°C for 10 min, followed by 35 cycles at 94°C for 30 s, 57°C for 30 s, and 72°C for 30 s, and a final elongation at 72°C for 10 min. The amplification protocol for c.3428A→G was similar, except for an annealing temperature of 60°C. Detection of the c.2243G→C mutation and the c.3428A→G polymorphism was performed by solid-phase minisequencing as described elsewhere (Suomalainen and Syvänen 2000), except that we used 0.1 μCi of the [3H]dNTPs (Amersham Biosciences). In brief, the biotinylated PCR product was bound to streptavidin-coated microtiter wells (Thermo Electron) and was denatured. A detection step primer was hybridized so that its 3′ end annealed just adjacent to the mutation site. A primer-extension reaction, with the use of one [3H]labeled dNTP and thermostable DNA polymerase (Dynazyme [Finnzymes]), was performed to identify the nucleotide at the mutation site. The detection primer was denatured, and the incorporated radioactivity was then measured in a liquid scintillation counter. The counts-per-minute values reflected the amount of the nucleotide present in the PCR product. The detection primer for c.2243G→C was 5′-TGTGAGGCAGCTTGAAAAAC-3′, and the radioactive nucleotides used to detect the mutation were [3H]dCTP (wild type) and [3H]dGTP (mutation). For c.3428A→G, we used detection primer 5′-CAGCGCGGTAGCGGTCCTCC-3′ and nucleotides [3H]dTTP (wild type) and [3H]dCTP (mutation).
The initial screening of the 306 ataxia samples for p.A467T (c.1399G→A in exon 7) was performed by a TaqMan 5′ nuclease assay by use of the forward and reverse primers 5′-CGGGAGATGAAGAAGTCGTTGATG-3′ and 5′-AAGGCCTGGCTACCTCTCT-3′, respectively; the normal and mutation-specific TaqMan MGB probes 5′-VIC-TCTGGCCAATGATG-3′ and 5′-FAM-ATCTGACCAATGATG-3′, respectively; and a nonfluorescent quencher at the 3′ end of each probe. The amplification and allelic discrimination were performed in an Mx3000P Real-Time PCR System (Stratagene). By use of TaqMan Universal PCR Mix (Applied Biosystems), 50 ng of DNA was amplified in 25 μl of buffer, and the PCR program included an initial cycle at 95°C for 10 min, followed by 42 cycles at 92°C for 15 s and 60°C for 1 min.
The remainder of the samples was screened for p.A467T by PCR and solid-phase minisequencing with methods similar to those for p.W748S and p.E1143G. The PCR primers used were 5′-Bio-ACCAGAACTGGGAGCGTTAC-3′ and 5′-CTACCTCTCTCCTGAGAGCA-3′, and the annealing temperature for PCR was 52°C. The detection primer was 5′-CAGCTGGCAGGCATCATTGG-3′, and the radioactive nucleotides were [3H]dCTP (wild type) and [3H]dTTP (mutation).
DNA Sequence Analysis
The exons of POLG1 were sequenced using intronic primers reported elsewhere (Van Goethem et al. 2001), by automated nucleotide sequencing with the BigDye terminator Ready Reaction Kit v.3.1 on the Applied Biosystems 3730 DNA Analyzer, as described by Luoma et al. (2004).
Haplotype Analyses
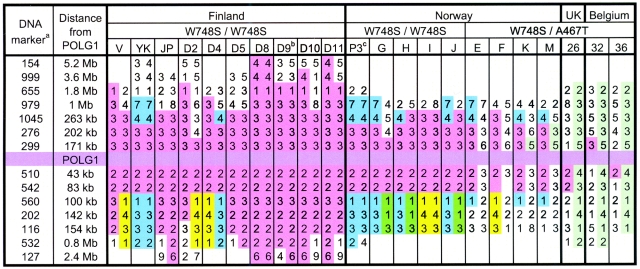
To determine the haplotypes of our patients with the W748S mutation, we analyzed 14 polymorphic dinucleotide markers flanking the POLG1 gene. The markers used were D15S154, D15S999, D15S655, D15S979, D15S1045, D15S276, D15S299, D15S510, D15S542, D15S560, D15S202, D15S116, D15S532, and D15S127.Figure 1 shows their distances from POLG1. PCR was performed in 0.2 mM of dNTPs, 0.33 μM of PCR primers, 0.8 μCi of [33P]dATP, and 1 U of thermostable DNA polymerase (Dynazyme [Finnzymes]), in 30 μl of buffer. The PCR program included an initial cycle at 94°C for 3 min, followed by 30–33 cycles at 94°C for 30 s, 48°C–62°C (optimized for each primer pair separately) for 30 s, and 72°C for 30 s, and a final elongation at 72°C for 10 min. The radioactive PCR products were electrophoresed through denaturing 6% polyacrylamide gels, and the film (Biomax MR films [Kodak]) was exposed by autoradiography for 1–2 d. In addition, intragenic SNPs were analyzed from the original sequencing data of the patient samples with the W748S mutation.
Figure 1.
Haplotypes of the Finnish, Norwegian, British, and Belgian patients. Haplotype A and the core haplotype are shown in pink. Haplotypes B and C are shown in blue and yellow, respectively. a, The DNA marker names are written without “D15S.” Proper names can be obtained by placing “D15S” in front of each number shown. b, Four Finnish patients with haplotypes very similar to that of patient D9 are not shown. c, One Norwegian patient with a haplotype very similar to that of patient P3 is not shown.
Bacterial Cloning of POLG1 Alleles
By bacterial cloning and sequence analysis of the resulting clones, we determined which SNPs were located in the same allele as W748S or E1143G. A PCR product containing exons 11–13 (with W748S and SNPs rs2072266 and rs2072267 [SNP numbers are from NCBI dbSNP]) was obtained using primers 5′-GAGTGGGCATCTGGTAATCA-3′ and 5′-TGTGGGCCTTGAGCAGAAT-3′, and a PCR product containing exons 19–21 (with E1143G and SNPs rs2246900 and rs2302084) was obtained using primers 5′-AGCCGTTTCTTCCTCTGA-3′ and 5′-CCAAAGCCCCACATAGGAGCACA-3′. PCR was performed in 0.2 mM dNTPs, 1.7 μM of PCR primers, and 0.5 U of DNA polymerase (Dynazyme EXT [Finnzymes]), in 30 μl of buffer. Standard cycling conditions were used, with an annealing temperature of 58°C. The PCR products were then cloned separately into pCR 2.1-TOPO vectors (Invitrogen), and the plasmid DNA was extracted from the clones with QIAprep Spin Miniprep Kit (Qiagen) and was subjected to sequence analysis as described above.
Results
DNA Analyses of Finnish Samples
We identified 27 Finnish patients with MIRAS, from 15 different families, on the basis of DNA analysis and the medical histories of the patients’ close relatives. In all these families, the patients were homozygous for the W748S and E1143G combination. One of the patients with MIRAS had been previously identified as a carrier of an SCA8 expansion, with no family history of SCA8.
Eleven of the patients were born in the 1950s, for a birth incidence of 1:81,500. On the basis of this incidence, the corresponding carrier frequency was calculated to be 1:140. Of the 500 control chromosomes analyzed, 2 chromosomes carried the W748S POLG mutation. Therefore, the carrier frequency of W748S was estimated to be 1:125. Of the 300 control chromosomes screened, 8 were carriers of the E1143G amino acid change, which gives an allele frequency of 2.7% and shows that E1143G is a frequent polymorphism, not always accompanied by W748S.
From the screened patient material, we also identified three patients heterozygous for W748S. Samples from these patients were forwarded for sequence analysis of the entire POLG1. However, no further mutations were identified. In addition, one patient was identified as heterozygous for the A467T mutation.
Clinical Manifestations
Because of the high number of patients identified as homozygous for W748S+E1143G, we studied the manifestations of the disease from medical records. Four typical patient histories have been reported elsewhere (Rantamäki et al. 2001; Van Goethem et al. 2004). The two patient case reports that follow, being two extremes of the disease manifestation, demonstrate the clinical heterogeneity of MIRAS.
Patient V
This patient was the fifth of five children of healthy, nonconsanguineous parents. Birth and early milestones were normal. At age 16 years, she had an eye infection and a slight head injury within a 1-wk interval. One week after the injury, she suddenly developed recurrent tonic-clonic seizures. At medical examination, she had a slight fever, she was mildly confused, her ability to maintain attention was reduced, and her relatives reported a change in personality. She also had symptoms suggesting a lesion of the left hemisphere: weakness of the right limbs and homonymous hemianopia. CT findings were normal. An electroencephalogram (EEG) showed slow wave activity with spikes bilaterally in the occipital region, with spreading to the left hemisphere. She was thought to have herpes encephalitis, even though she lacked herpes antibodies and EEG findings typical for that condition. EEG background abnormalities and some neuropsychological changes persisted thereafter, but the symptoms indicating a hemispheric lesion resolved. Despite anticonvulsive medication, she continued to have epileptic seizures. She also had decreased deep-tendon reflexes in the lower extremities but only mild defects of coordination. Magnetic resonance imaging (MRI) at age 23 years showed symmetric, bilateral high-signal-intensity lesions in the cerebellum and thalamus. She developed gait and limb ataxia and dysarthria, which were prominent at age 31 years. In addition, she had some choreoathetoid movements, and her cognitive impairment had progressed: she had childlike conversational skills, and neuropsychological tests showed impairments of memory and visual reasoning. Muscle strength, vibration sense, and touch sensation were all normal. At age 34 years, she was using a wheelchair and walked short distances with a walker. Her involuntary movements had aggravated. Epileptic seizures gradually became frequent, and, at age 37 years, she was hospitalized permanently. In the hospital, she had dysphagia and was paranoid, and her cooperation and general condition gradually worsened. The patient’s sister also suffered from seizures and cognitive decline, with a similar onset of symptoms, and she died at age 31 years. At the time, the sister’s symptoms were assumed to be a result of encephalitis in a postencephalitic state. The three other sisters were unaffected at 39, 38, and 35 years of age. The patient’s cousin had died at age 45 years and was diagnosed with possible Leigh disease on the basis of neuropathological findings.
Patient D10
At age 45 years, this previously healthy man sought medical advice because of a feeling of numbness and weakness in the lower extremities when walking. The symptoms had gradually worsened during 3–4 years. He also complained of memory loss during the previous year. At examination, muscle strength was normal, vibration sense was slightly impaired in the lower extremities, and deep-tendon reflexes were absent in the lower extremities but normal in the upper limbs. Romberg test was negative, and heel-to-shin coordination test was normal, but the patient had some problems with balance when standing on one leg. A moderate sensory-motor polyneuropathy was diagnosed by electroneuromyography (ENMG). CT showed mild cerebellar atrophy and hypodense changes in both cerebellar hemispheres. At age 54 years, his balance problems had gradually aggravated, he was suffering from frequent falls, and he had dysarthria. Mild atrophy of the cerebellum was seen on brain CT. He was then diagnosed with cerebellar ataxia and cognitive impairment, in addition to the previously diagnosed polyneuropathy. He was also obese and had hyperlipidemia. Psychiatric symptoms, hallucinations, and paranoia were later manifestations of the disease after age 57 years.
Patients overall
Table 1 summarizes the clinical features of 19 patients from 14 different families. Four of the patients (F2, III-7, III-5, and III-4) have been reported elsewhere (Rantamäki et al. 2001; Van Goethem et al. 2004). The median age at onset of MIRAS was 28 years; the range was 5–41 years. Progressive gait unsteadiness was the most common first symptom of the disease. These patients had been experiencing balance problems for several years before the first medical examination. They usually presented with sensory ataxia and either decreased or absent deep-tendon reflexes in the lower extremities. Besides gait unsteadiness, epilepsy and neuropathy (i.e., neuropathic sensations) were other common initial features of MIRAS, and these sometimes occurred before the onset of gait unsteadiness. In a few cases, epilepsy preceded other prominent symptoms by >10 years.
Table 1.
Clinical Features of Finnish Patients Homozygous for the W748S Mutation[Note]
| Patient | |||||||||||||||||||
| Clinical Feature | D4a | V | D2 | D2S | D6S | D6 | D11 | D7 | D10 | D5 | D3 | D1 | D1S | D8 | 121 | F2b | III-7c | III-5c | III-4c |
| Age (years) | 39 | 37 | 51 | 35d | 46 | 44 | 42 | 38 | 58 | 51 | 44 | 45 | 45 | 51 | 55 | 33 | 36d | 48 | 51 |
| Sex | M | F | F | M | M | F | F | M | M | F | M | M | M | M | F | M | F | F | M |
| Age at onset (years) | 27 | 16 | 28 | 5 | 32 | 19 | 27 | 27 | 41 | 36 | 38 | 17 | 23 | 38 | 24 | 30 | 29 | 30 | 30 |
| First symptoms | BD | Epi | HA | Tr | BD | Epi | BD | NP, BD | NP | NP | BD, NP | Epi | Epi | BD, NP | Epi | BD | BD | BD | Psych, BD |
| Gait and limb ataxia | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| ENMG-verified NP | + | NA | NA | NA | + | + | + | + | + | + | + | NA | + | + | + | + | + | + | + |
| Tendon reflexes in lower limbs | D | D | D | D | A | A | A | A | A | A | A | A | A | D | A | A | D | A | A |
| Babinski sign | id/id | −/− | −/− | +/+ | −/− | −/− | id/id | −/− | −/− | −/− | −/− | −/− | −/− | NA | −/− | −/− | +/+ | −/− | −/− |
| Decreased vibration or joint position sense | + | − | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Epilepsy | − | + | + | + | − | + | − | − | − | − | − | + | + | − | + | − | + | − | + |
| Myoclonus | − | + | + | + | − | + | − | − | − | − | − | + | + | + | − | − | − | − | − |
| Involuntary movements | Ath | Ath, Face | + | Tr | − | Ath | Face | Tr, Ath | − | Tr | − | hTr, Face | hTr | Tr | − | − | hTr | hTr | − |
| Dysarthria | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Dysphagia | − | + | + | − | + | + | + | + | + | − | − | − | − | − | − | + | + | + | − |
| Nystagmus | + | (+) | + | − | + | + | + | (+) | − | + | − | (+) | + | − | + | − | + | + | + |
| Other eye-movement abnormalities | R | O | R, O, P | W | Dipl | O | O | R | O | − | R, P | O | O | R | Dipl | O | Dipl, O | R, O, P | R, O |
| Cognitive impairment | (+) | + | + | + | + | + | − | − | + | − | + | + | + | + | − | − | + | + | + |
| Psychiatric symptoms | − | + | + | + | − | − | (+) | + | + | (+) | − | (+) | (+) | + | + | − | − | − | + |
| Muscle strength | N | D | D | D | D | N | N | D | N | D | N | N | N | N | N | NA | N | N | D |
| Obesity | + | + | + | NA | − | + | + | + | + | NA | − | − | − | + | NA | + | + | + | − |
| Other symptoms | … | … | … | … | HD | HD | Men | Amy, PC, Cr | … | Amy, PC | Cr | Cr | Cr | … | … | Cr | … | … | … |
Note.— Siblings are designated with an “S” at the end of the patient number. + = finding present; (+) = suspected finding; − = finding not present; A = absent; Amy = amyotrophy; Ath = athetoid or choreoathetoid; BD = balance disturbances; Cr = muscle cramps; D = decreased; Dipl = diplopia; Epi = epilepsy; Face = facial involuntary movements; HA = headache; HD = sensory neural hearing deficit; hTr = head tremor; id/id = indifferent/indifferent; Men = premature menopause; N = normal; NA = not available; NP = neuropathy; O = other eye-movement abnormalities (ataxia, supranuclear eye-movement abnormalities, saccadic eye movements, or slowed or jerky ocular pursuit movements); P = ptosis; PC = pes cavus; Psych = psychiatric symptoms; R = restricted eye movements; Tr = tremor; W = eye muscle weakness.
Patient with an SCA8 repeat expansion.
This patient has been reported elsewhere (Van Goethem et al. 2004).
Patient's age at time of death.
Symptoms suggesting cerebellar dysfunction, such as ataxia, dysarthria, nystagmus, or other eye-movement abnormalities, were sometimes noticeable at the first examination, whereas, in other cases, they developed later. Eye-movement abnormalities were typically mild but included ataxic, saccadic, or nystagmoid eye movements; supranuclear eye-movement abnormality; and restricted eye movements suggestive of central origin. All patients developed ataxia and features of cerebellar speech (dysarthria in all patients and impaired speech volume control in four patients). Muscle strength was typically good until the late stages of the disease.
The cognitive impairment observed in these patients was mild to moderate. The psychiatric symptoms ranged from denial of symptoms to severe depression, anxiety disorder, aggressivity, hallucinations, and paranoia. Involuntary movements were described as choreoathetoid movements of the limbs, myoclonus, head tremor, tremor of the limbs (also at rest), and involuntary facial movements.
Neuropathic pain was experienced by some of the patients. ENMG was performed for 15 patients, and they all had moderate to severe sensory neuropathy in the lower extremities. In addition, the sensory nerves of the upper limbs and the motor nerves of the lower extremities were also slightly affected in most cases. The neuropathy was mostly of the axonal type. All four patients who did not undergo ENMG had decreased or absent deep-tendon reflexes in the lower extremities.
Of the 19 patients, 18 underwent at least one brain MRI or CT. Twelve patients had bilateral white-matter changes in the cerebellar hemispheres at age 23–49 years: high-signal-intensity lesions on MRI or hypodense lesions on CT. Five patients without these lesions had at least minor infratentorial, cerebellar, or vermis atrophy. Altogether, atrophy was suspected in 16 patients. Thalamic lesions were seen on the MRI of seven patients. Few patients had cortical atrophy or enlarged lateral ventricles.
Muscle was biopsied from eight patients, and features typical of denervation were present in some of the patients. Most often, muscle biopsy and its histological analyses or lactate measurements were not done, since the disease resembled SCAs, not mitochondrial diseases. None of the analyzed samples showed morphological changes clearly suggestive of a mitochondrial disease. Results from histochemical activities of cytochrome oxidase, succinate dehydrogenase, and nicotinamide adenine dinucleotide–tetrazolium reductase were available for three, two, and one patients, respectively. The findings did not support the diagnosis of a mitochondrial disease, except in one patient, who had few fibers of ragged-red type. Results of long-range PCR have been reported and discussed elsewhere (Van Goethem et al. 2004); low amounts of mtDNA deletions were seen in the muscle of two patients. The lactate levels in the cerebrospinal fluid or blood were occasionally marginally elevated but were not measured in most patients. The EEG findings were abnormal for 11 patients, who all had background EEG abnormalities, and were normal for 1 patient.
Haplotype Analysis
Figure 1 shows the results of the haplotype analysis. All the Finnish patients shared a short, common core haplotype of four polymorphic dinucleotide markers, extending over a chromosomal region of ∼280 kb. However, the shared homozygous haplotype extended >7.6 Mb in some patients. Outside the common core region, three different distal haplotypes with DNA marker alleles in linkage disequilibrium (LD) could be identified in the Finnish patients for markers D15S560–D15S532: haplotype A with 3-2-2-2/1, haplotype B with 1-3-3-2, and haplotype C with 1-4-3-1. Haplotype A was found in the majority of chromosomes: altogether, in 24 of the 31 chromosomes analyzed. Haplotypes B and C were seen in three and four chromosomes, respectively.
Figure 2 shows the birthplaces of the grandparents of the Finnish patients (data available for 41 grandparents). Grandparents of patients with haplotype A have birthplaces concentrated in the late-settlement area, the part of Finland that became permanently inhabited only in the 16th century. Grandparents of patients with haplotypes B and C have birthplaces solely on the western and southern coastlines, respectively. The genealogical study reaching back to 1850 revealed no recent common ancestors between the patients.
Figure 2.
Birthplaces of the grandparents of the Finnish patients homozygous for the W748S POLG mutation, on a map of Finland. Haplotype A is concentrated in the late-settlement area, whereas haplotypes B and C cluster solely on the western and southern coastlines. The southeastern Karelia belongs presently to Russia and is therefore indicated as separate on the map.
In addition to the Finnish homozygotes for W748S from 14 different families and the three Finnish heterozygotes for W748S, we analyzed the haplotypes of 10 Norwegian patients, two Belgian patients (patients 32 and 36), one British patient (patient 26), all carrying at least one allele of W748S+E1143G. All the Norwegian patients shared the core haplotype with the Finns, over a region of >200 kb (fig. 1). Haplotype B, also found in the Swedish-speaking population of the Finnish west-coast, was the major haplotype in Norwegian patients, with seven identified chromosomes. Some remnants of haplotype B were seen in three chromosomes, and haplotype C was seen in three chromosomes. An additional amino acid variant in POLG, Q497H, cosegregated with haplotype B in both the Norwegian and the Finnish patients. Since remnants of the Finnish haplotypes were also seen in the dinucleotide marker analysis of the Belgian and British patients, we completed the data with intragenic SNPs. Figure 1 and table 2 show the dinucleotide and SNP haplotypes of the European patients, respectively. One allele of the British and Belgian patients shared the intragenic SNP haplotype and the immediate gene vicinity with the Finns and the Norwegians. In the second allele, the Belgian and British patients shared a common haplotype of ∼2 Mb, which was different from that of the Finns. By allele-specific cloning and sequencing, we showed that the G (rs2246900) and C (rs2302084) actually resided in the same allele with E1143G, and that C (rs2072266) and C (rs2072267) were found in the same allele with W748S in the British patient. These data strongly indicate that the short core SNP haplotype is associated with the W748S+E1143G allele, whereas the 2-Mb common allele is associated with the A467T mutation, providing evidence that the British and Belgian disease chromosomes originate from the same common founder as the disease chromosomes of the Finns and Norwegians.
Table 2.
SNP Haplotypes of Finnish, British, and Belgian Patients[Note]
| SNP | Intron | Frequencya | Finnish | Britishb | Belgianc |
| rs2307438 | 21 | .463 | G/G | T/G | G/G |
| E1143G | … | .026 | G/G | G/A | G/A |
| rs2302084 | 19 | .336 | C/C | C/T | C/T |
| rs2246900 | 19 | .305 | G/G | G/A | G/A |
| rs2307449 | 18 | .463 | C/C | C/A | C/A |
| rs2307433 | 17 | .548 | Ins/Ins | Ins/Del | Ins/Del |
| W748S | … | … | C/C | C/G | C/G |
| rs2072266 | 12 | .361 | C/C | C/T | C/Td |
| rs2072267 | 11 | .54 | C/C | C/T | C/T |
| rs3176183 | 9 | .323 | Ins/Ins | Ins/Del | Ins/Del |
Note.— Bold typeface emphasizes the similarity between patients. Del = deletion; Ins = insertion.
Allele frequencies according to the SNPs of the Finnish patient.
Patient 26.
Patients 32 and 36.
Data available only for patient 32.
Discussion
We demonstrate here that defective mtDNA polymerase is a frequent cause of recessive ataxia, with a carrier frequency of 1:125 in Finland. We detected a clear founder effect with the W748S mutation allele in Finland, but the Finnish core haplotype was also shared by patients from Norway, the United Kingdom, and Belgium. The common ancient European origin of the disease chromosome suggests that this is a frequent cause of recessive ataxia in Europe and not solely restricted to and enriched in Finland. We have named the POLG1-associated ataxia syndromes “MIRAS” (which denotes “mitochondrial recessive ataxia syndrome”).
Our initial findings of a few patients who have ataxia with the W748S mutation (Van Goethem et al. 2004) prompted us to analyze all those samples that had been sent for SCA DNA diagnostic testing during the years 1993–2004. These samples represented all of Finland, with a population of 5.2 million, since the SCA analyses were concentrated at a single diagnostic center in the country. We identified altogether 15 families, with 27 patients, and determined the carrier frequency to be 1:125. This correlated well with the calculated birth incidence, 1:81,500. To date, SCA8 and SCA7 have been the most prevalent SCAs in Finland, with nine and six autosomal dominant families described, respectively (Juvonen et al. 2002, 2005), and infantile-onset spinocerebellar ataxia (IOSCA) has been the most common pediatric form, with 22 patients described (Koskinen et al. 1994; T. Lönnqvist, personal communication). The most common hereditary ataxia worldwide, Friedreich ataxia (Harding 1981; Durr et al. 1996), is rare in Finland, with seven identified patients. Our results establish MIRAS as the most prevalent juvenile- or adult-onset ataxia type in Finland.
To study the population history of the newly identified disease, we determined the haplotypes of the Finnish patients with MIRAS. The mutation was invariably associated with the E1143G variant—a frequent polymorphism with an allele frequency of 3% in Finns. The question of whether E1143G has a functional effect, synergistic with or antagonistic to W748S, awaits functional studies. All the Finnish patients homozygous for W748S+E1143G allele shared a short core haplotype of ∼280 kb, which suggests an ancient common founder for the mutation. Those diseases belonging to the Finnish disease heritage (Norio et al. 1973; Norio 2003) that share a haplotype of 2–3 Mb date back to the first bottleneck of the Finnish population in the first millennium (Varilo 1999). This suggests that the common ancestor with MIRAS for Finnish haplotypes most likely lived >2,000 years ago. Furthermore, outside the common chromosomal core region, three major haplotypes with different alleles in LD with the core region extend to cover a region of ∼10 cM. This is exceptional for a Finnish disease, in which usually one major disease haplotype has spread within Finland from a single founder. The three long areas of LD suggest that the MIRAS disease chromosomes may have been introduced to the Finnish population on three independent occasions, each occurring rather recently, ∼10–30 generations ago (Varilo 1999), with subsequent enrichment of separate disease alleles within the country. This is supported by the localization of the birthplaces of the patients’ grandparents to different sites of the country. In brief, the introduction of the disease chromosomes to the Finnish population on three different occasions is supported by: (1) LD extending over a similar chromosomal distance in all three Finnish haplotypes, suggesting a similar age for all the haplotypes, (2) the relatively small amount of haplotypes B and C, indicating that neither of these haplotypes gave rise to the other haplotypes, and (3) the absence of any intermediate haplotypes (with only a telomeric or centromeric recombination) that would support the evolution of one haplotype to another within Finnish borders.
The distant ancestors of the Finnish patients prompted us to look at non-Finnish patients. The POLG W748S mutation has been recently described in Italian patients with Alpers syndrome, as well as in British and Norwegian families with ataxia phenotypes (Van Goethem et al. 2004; Ferrari et al. 2005; Winterthun et al. 2005), usually occurring as a compound heterozygous recessive mutation. All these chromosomes also carried the E1143G mutation. We studied the associated haplotypes of Finnish, British, Belgian, and Norwegian patients and identified a short, common intragenic SNP haplotype in all these samples, which suggestively continued to the immediately adjacent chromosomal region. These results strongly suggest that all our European cases have a common ancient founder. Interestingly, the Finnish west-coast haplotype, encountered in the Swedish-speaking Finnish population, had at least a 1.2-Mb region of identity with the major haplotype in Norway, which suggests a relatively recent relocation of a Norwegian or other Scandinavian ancestor to Finland. These chromosomes also carried a third POLG amino acid variant, Q497H, which was absent in the other haplotypes. These data suggest migration of the founder population within Scandinavia. Furthermore, the common core haplotype of the Finns and Norwegians was considerably longer than the shared haplotype of the western European samples, indicating a later branching of the northern haplotypes from the ancient European ancestor. The common founder in western and northern Europe and the high number of Norwegian patients suggest that W748S+E1143G is a common cause of ataxia in Europe.
In our non-Finnish patients, the W748S allele was often compound heterozygous with another POLG spacer-domain mutation, A467T. This amino acid variant occurred with an allele frequency of 0.6% in the Belgian population (Van Goethem et al. 2003a) and <0.2% in the Finnish population (Luoma et al. 2005). Interestingly, we saw a common A467T haplotype of ∼2 Mb in the British and Belgian patients, who were not known to be related. Remnants of this haplotype were also seen in two Norwegian patients but not in the other two Norwegians with the same mutation. Our limited material did not allow us to conclude whether the A467T allele would have spread from an ancient founder over Europe. Further studies on larger groups of patient are warranted.
Mitochondrial diseases are known for their clinical heterogeneity. However, this is most often related to mtDNA mutations and is explained by variable loads of mutant mtDNA in different tissues and patients. In the case of MIRAS, all our patients were homozygous for the same recessive mutation in the nuclear POLG1 gene, but the phenotype was still surprisingly variant, resembling the variance in diseases caused by an mtDNA mutation. The onset of the disease was most often at ∼30 years of age, with sensory axonal polyneuropathy or balance disturbances, followed by progressive cerebellar ataxia. Epilepsy and cognitive defects, sometimes accompanied by psychiatric symptoms, were often but not always present. The progression of the disease varied, but occasionally it was quite rapid, necessitating permanent hospitalization before age 50 years. Sometimes, the symptoms started already in childhood or adolescence and manifested with an acute incident of epilepsy. Epilepsy could be the only symptom in a patient for years, sometimes developing to treatment-resistant grand mal seizures, recurrent status epilepticus, and death. Also in such cases, ataxia manifested during the late 3rd decade of life. The mildest manifestation of the same homozygous W748S allele was sensory polyneuropathy, developing during the 5th decade of life. In the later course of the disease, the clinical symptoms and signs of different patients were alike, with the exception of epilepsy, which was seen in 50% of cases. The abnormal eye movements were not typical for external ophthalmoplegia but were mainly coordination problems of central origin. The MRI findings, initially described in our previous reports of the index patients (Rantamäki et al. 2001; Van Goethem et al. 2004), were established here as typical for the disease. Most cases showed symmetrical lesions of high signal intensity in the white matter of the cerebellum and minor atrophy of the cerebellum or the vermis, sometimes combined with symmetrical high-signal lesions of the thalami. Mitochondrial disease was sometimes suspected on the basis of clinical findings, but characteristic histological and biochemical findings were lacking. In one patient, coexistence of an SCA8 repeat expansion and homozygosity for the W748S mutation did not act synergistically; the patient’s disease was typical for MIRAS. Overall, MIRAS with the homozygous W748S+E1143G mutation has typical clinical features but shows significant variability uncommon for an autosomal recessive disorder, leading to misdiagnoses of, for example, Huntington chorea, Leigh disease, hereditary neuropathy, or herpes encephalitis.
The clinical features of MIRAS are indistinguishable from the disease group of autosomal dominant cerebellar ataxias (ADCAs), known as “SCAs” in genetic nomenclature. Gait and limb ataxia, dysarthria, polyneuropathy, hyporeflexia, nystagmus, cognitive impairment, and epilepsy are all clinical features of ADCAs (Harding 1983; Schöls et al. 2004). Clinical symptoms of MIRAS most closely resemble two other recessively inherited SCAs, Friedreich ataxia and IOSCA, although MIRAS has a later onset. Table 3 summarizes the clinical features of these three ataxias (Harding 1981; Koskinen et al. 1994; Durr et al. 1996). Friedreich ataxia is caused by a defect in frataxin, a mitochondrial protein involved in iron homeostasis (Campuzano et al. 1996; Foury and Cazzalini 1997). IOSCA is a severe infantile hereditary ataxia, which has been recently associated with a mutation in the mitochondrial replicative helicase Twinkle (K. Nikali, A. Suomalainen, J. Saharinen, M. Kuokkanen, J. N. Spelbrink, T. Lönnqvist, and L. Peltonen, unpublished data). This finding links IOSCA closely to the same pathogenetic mechanism as MIRAS—that is, mtDNA maintenance. In IOSCA and MIRAS, histological abnormalities suggestive of mitochondrial dysfunction were not seen in the patients’ muscle samples, and no mtDNA deletions were detected by Southern blot analysis. So far, no data about mtDNA mutations in the brain have been available for these syndromes. The two proteins of mtDNA maintenance, POLG and Twinkle, seem to have a crucial role in the survival of cerebellar neurons, and defects of these proteins are common causes of hereditary ataxia. Furthermore, the similarity between the three mitochondrial recessive ataxia types—Friedreich ataxia, IOSCA, and MIRAS—suggests that they form a new important group of hereditary ataxias.
Table 3.
Clinical Features of Three Autosomal Recessive Ataxias: MIRAS, Friedreich Ataxia, and IOSCA[Note]
| Clinical Feature | MIRAS | FriedreichAtaxia | IOSCA |
| Age at onset (years) | 5–50 | <25a | 2–3 |
| Ataxia | + | + | + |
| Cerebellar dysarthria | + | + | + |
| Lower-limb areflexia | + | + | + |
| Sensory axonal neuropathy | + | + | + |
| Cognitive impairment | + | (+) | + |
| Pes cavus | (+) | + | + |
| Involuntary movements: | |||
| Athetosis | (+) | − | + |
| Myoclonus | + | (+) | − |
| Extensor plantar response | (+) | + | + |
| Muscle weakness | (+) | + | + |
| Epilepsy | + | − | + |
| Cardiomyopathy, scoliosis, diabetes | − | + | − |
| Ophthalmoplegia | (+) | − | + |
| Sensorineural hearing loss | (+) | (+) | + |
| Optic atrophy | − | (+) | + |
Note.— + = present; (+) = present in only a few patients; − = not present.
Age at onset for atypical cases is 25–51 years.
In conclusion, we have provided here comprehensive genealogical and clinical details for a newly identified hereditary ataxia. The Finnish MIRAS phenotype was surprisingly variant, ranging from late-onset polyneuropathy to acute encephalopathy of adolescence. This could be the result of individual differences in defence mechanisms against cellular stresses, mtDNA haplotypes, or variants in the POLG-associated proteins, and it remains to be studied in detail. The high carrier frequency of the disease in Finland, the high number of patients in Norway, and the ancient European founder chromosome indicate that MIRAS should be considered for first-line genetic testing of patients who have ataxia with a suspected recessive disease. Furthermore, our data strongly suggest that mutations in POLG1 are emerging as an important general cause of recessively inherited ataxias of the spinocerebellar type.
Acknowledgments
We thank the patients and their family members, for their cooperation. We also thank their doctors, for providing the medical records. Dr. Pentti Tienari is thanked for his comments on the manuscript, and Dr. Wim Robberecht, for patient referral. We thank the following organizations for financial support: the University of Helsinki (to A.S.); the Academy of Finland, Sigrid Juselius Foundation (to A.S. and K.M.); the clinical EVO grants of South Ostrobotnia Hospital District (to M.R.); The Vaasa Central Hospital Medical Research Fund (to B.U.); L. Meltzer Høyskolefond (project 480210), and Helse Vest, Norway (to L.A.B.).
Web Resources
Accession numbers and URLs for data presented herein are as follows:
- dbSNP, http://www.ncbi.nlm.nih.gov/SNP/ (for SNPs rs2307438, rs2302084, rs2246900, rs2307449, rs2307433, rs2072266, rs2072267, and rs3176183)
- GenBank, http://www.ncbi.nlm.nih.gov/Genbank/ (for POLG1 [accession number NM_002693]; sequence numbering starts from ATG)
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for POLG1; PEO; SCA1, 2, 3, 6, 7, 8, 10, 12, and 17; DRPLA; and FRDA)
References
- Campuzano V, Montermini L, Molto MD, Pianese L, Cossee M, Cavalcanti F, Monros E, et al (1996) Friedreich’s ataxia: autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science 271:1423–1427 [DOI] [PubMed] [Google Scholar]
- Durr A, Cossee M, Agid Y, Campuzano V, Mignard C, Penet C, Mandel JL, Brice A, Koenig M (1996) Clinical and genetic abnormalities in patients with Friedreich’s ataxia. N Engl J Med 335:1169–1175 [DOI] [PubMed] [Google Scholar]
- Ferrari G, Lamantea E, Donati A, Filosto M, Briem E, Carrara F, Parini R, Simonati A, Santer R, Zeviani M (2005) Infantile hepatocerebral syndromes associated with mutations in the mitochondrial DNA polymerase-γA. Brain 128:723–731 [DOI] [PubMed] [Google Scholar]
- Foury F, Cazzalini O (1997) Deletion of the yeast homologue of the human gene associated with Friedreich’s ataxia elicits iron accumulation in mitochondria. FEBS Lett 411:373–377 [DOI] [PubMed] [Google Scholar]
- Harding AE (1981) Friedreich’s ataxia: a clinical and genetic study of 90 families with an analysis of early diagnostic criteria and intrafamilial clustering of clinical features. Brain 104:589–620 [DOI] [PubMed] [Google Scholar]
- ——— (1983) Classification of the hereditary ataxias and paraplegias. Lancet 1:1151–1155 [DOI] [PubMed] [Google Scholar]
- Juvonen V, Hietala M, Kairisto V, Savontaus ML (2005) The occurrence of dominant spinocerebellar ataxias among 251 Finnish ataxia patients and the role of predisposing large normal alleles in a genetically isolated population. Acta Neurol Scand 111:154–162 [DOI] [PubMed] [Google Scholar]
- Juvonen V, Kairisto V, Hietala M, Savontaus ML (2002) Calculating predictive values for the large repeat alleles at the SCA8 locus in patients with ataxia. J Med Genet 39:935–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaguni LS (2004) DNA polymerase γ, the mitochondrial replicase. Annu Rev Biochem 73:293–320 [DOI] [PubMed] [Google Scholar]
- Koskinen T, Santavuori P, Sainio K, Lappi M, Kallio AK, Pihko H (1994) Infantile onset spinocerebellar ataxia with sensory neuropathy: a new inherited disease. J Neurol Sci 121:50–56 [DOI] [PubMed] [Google Scholar]
- Lamantea E, Tiranti V, Bordoni A, Toscano A, Bono F, Servidei S, Papadimitriou A, Spelbrink H, Silvestri L, Casari G, Comi GP, Zeviani M (2002) Mutations of mitochondrial DNA polymerase γA are a frequent cause of autosomal dominant or recessive progressive external ophthalmoplegia. Ann Neurol 52:211–219 [DOI] [PubMed] [Google Scholar]
- Longley MJ, Prasad R, Srivastava DK, Wilson SH, Copeland WC (1998) Identification of 5′-deoxyribose phosphate lyase activity in human DNA polymerase γ and its role in mitochondrial base excision repair in vitro. Proc Natl Acad Sci USA 95:12244–12248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luoma P, Melberg A, Rinne JO, Kaukonen JA, Nupponen NN, Chalmers RM, Oldfors A, Rautakorpi I, Peltonen L, Majamaa K, Somer H, Suomalainen A (2004) Parkinsonism, premature menopause, and mitochondrial DNA polymerase γ mutations: clinical and molecular genetic study. Lancet 364:875–882 [DOI] [PubMed] [Google Scholar]
- Luoma PT, Luo N, Löscher WN, Farr CL, Horvath R, Wanschitz J, Kiechl S, Kaguni LS, Suomalainen A (2005) Functional defects due to spacer region mutations of human mitochondrial polymerase in a family with an ataxia-myopathy syndrome. Hum Mol Genet 14:1907–1920 [DOI] [PubMed] [Google Scholar]
- Naviaux RK, Nguyen KV (2004) POLG mutations associated with Alpers’ syndrome and mitochondrial DNA depletion. Ann Neurol 55:706–712 [DOI] [PubMed] [Google Scholar]
- Norio R (2003) Finnish disease heritage. I. Characteristics, causes, background. Hum Genet 112:441–456 [DOI] [PubMed] [Google Scholar]
- Norio R, Nevanlinna HR, Perheentupa J (1973) Hereditary diseases in Finland: rare flora in rare soul. Ann Clin Res 5:109–141 [PubMed] [Google Scholar]
- Rantamäki M, Krahe R, Paetau A, Cormand B, Mononen I, Udd B (2001) Adult-onset autosomal recessive ataxia with thalamic lesions in a Finnish family. Neurology 57:1043–1049 [DOI] [PubMed] [Google Scholar]
- Schöls L, Bauer P, Schmidt T, Schulte T, Riess O (2004) Autosomal dominant cerebellar ataxias: clinical features, genetics, and pathogenesis. Lancet Neurol 3:291–304 [DOI] [PubMed] [Google Scholar]
- Shibata DK, Arnheim N, Martin WJ (1988) Detection of human papilloma virus in paraffin-embedded tissue using the polymerase chain reaction. J Exp Med 167:225–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suomalainen A, Syvänen AC (2000) Quantitative analysis of human DNA sequences by PCR and solid-phase minisequencing. Mol Biotechnol 15:123–131 [DOI] [PubMed] [Google Scholar]
- Syvänen AC, Aalto-Setala K, Harju L, Kontula K, Soderlund H (1990) A primer-guided nucleotide incorporation assay in the genotyping of apolipoprotein E. Genomics 8:684–692 [DOI] [PubMed] [Google Scholar]
- Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, Bruder CE, Bohlooly-Y M, Gidlöf S, Oldfors A, Wibom R, Törnell J, Jacobs HT, Larsson NG (2004) Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 429:417–423 [DOI] [PubMed] [Google Scholar]
- Van Goethem G, Dermaut B, Löfgren A, Martin JJ, Van Broeckhoven C (2001) Mutation of POLG is associated with progressive external ophthalmoplegia characterized by mtDNA deletions. Nat Genet 28:211–212 [DOI] [PubMed] [Google Scholar]
- Van Goethem G, Luoma P, Rantamäki M, Al Memar A, Kaakkola S, Hackman P, Krahe R, Löfgren A, Martin JJ, De Jonghe P, Suomalainen A, Udd B, Van Broeckhoven C (2004) POLG mutations in neurodegenerative disorders with ataxia but no muscle involvement. Neurology 63:1251–1257 [DOI] [PubMed] [Google Scholar]
- Van Goethem G, Martin JJ, Dermaut B, Löfgren A, Wibail A, Ververken D, Tack P, Dehaene I, Van Zandijcke M, Moonen M, Ceuterick C, De Jonghe P, Van Broeckhoven C (2003a) Recessive POLG mutations presenting with sensory and ataxic neuropathy in compound heterozygote patients with progressive external ophthalmoplegia. Neuromuscul Disord 13:133–142 [DOI] [PubMed] [Google Scholar]
- Van Goethem G, Mercelis R, Löfgren A, Seneca S, Ceuterick C, Martin JJ, Van Broeckhoven C (2003b) Patient homozygous for a recessive POLG mutation presents with features of MERRF. Neurology 61:1811–1813 [DOI] [PubMed] [Google Scholar]
- Varilo T (1999) The age of the mutations in the Finnish disease heritage: a genealogical and linkage disequilibrium study. PhD thesis, National Public Health Institute and University of Helsinki, Helsinki. Available at: http://ethesis.helsinki.fi/english.html
- Winterthun S, Ferrari G, He L, Taylor RW, Zeviani M, Turnbull DM, Engelsen BA, Moen G, Bindoff LA (2005) Autosomal recessive mitochondrial ataxic syndrome due to mitochondrial polymerase gamma mutations. Neurology 64:1204–1208 [DOI] [PubMed] [Google Scholar]
- Zeviani M, Servidei S, Gellera C, Bertini E, DiMauro S, DiDonato S (1989) An autosomal dominant disorder with multiple deletions of mitochondrial DNA starting at the D-loop region. Nature 339:309–311 [DOI] [PubMed] [Google Scholar]